15
Renal and intestinal vascular disease
Renal artery disease
Fibromuscular dysplasia (FMD)
FMD is a non-inflammatory, non-atherosclerotic disorder that may be observed in almost any arterial bed and can lead to arterial stenosis. Five different types are recognised and usually affect younger patients, with a female predominance, involving the distal main artery and/or the intrarenal branches. Rarely, FMD may be complicated by an aneurysm. Patients may be asymptomatic but the most usual presentation is hypertension. Hypertension is commonly treated successfully with medication, but renal artery stenosis and renal dysfunction may progress in up to one-third of patients. Occlusion and complete loss of renal function is exceptional. Magnetic resonance angiography (MRA) can detect FMD in the proximal vessels, but is less sensitive for visualising the second- and third-order branches. The diagnosis will usually require conventional digital subtraction angiography and selective views may be necessary to detect subtle branch lesions. When treating FMD, the results of percutaneous angioplasty (PTA) are good, with 10-year cumulative patency rates of 87% and up to 50% of patients cured of their hypertension. The remainder have a reduced drug burden and improved blood pressure control.1 Stenting is usually reserved for suboptimal PTA.
Arteritis
Involvement of the renal artery is not uncommon in systemic vasculitis. The diagnosis can be difficult and in the absence of other systemic symptoms may require a renal biopsy. Takayasu’s arteritis (see also Chapter 12) is a non-specific inflammatory disease that mainly affects large arteries such as the aorta and its main branches including the renal artery, and is the most common cause of renovascular hypertension in India and China. The majority of patients can be managed medically on corticosteroids, with monitoring of disease activity using the erythrocyte sedimentation rate (ESR). In the chronic inactive stage, PTA can produce a reasonable blood pressure response.2
Atherosclerotic renal vascular disease
Atherosclerotic stenosis is the most common pathological condition of the renal arteries, affecting 1–5% of patients with hypertension.3 Occlusion of the renal artery is present in up to 50% of patients so the condition is best described as atherosclerotic renovascular disease (ARVD) rather than as renal artery stenosis (RAS). Autopsy data demonstrate that the incidence of ARVD increases with age, affecting 18% of individuals between the ages of 65 and 74 years and > 40% of those over 75 years.4 Medicare data from America indicate that ARVD has an incidence of 3.9 cases per 1000 of the population over 65 years.5 Usually, ARVD is a manifestation of generalised atherosclerosis involving multiple vessels.
Widespread atheroma is usually present in the aorta and typically compromises the proximal 1–2 cm of one or both renal arteries. If untreated, atherosclerotic stenosis progresses to renal artery occlusion in up to 50% of patients6 and permanent loss of the renal parenchyma in 15–25% of patients, particularly in the elderly.7 The exact level of angiographic stenosis that represents significant ARVD is controversial and varies between 50% and 75% in diameter loss. Large-animal experiments have demonstrated a significant pressure gradient across a stenosis of 60%;8 however, in humans a lesion even less than 50% can be associated with gradients of 15 mmHg.9
Pathophysiology
Renal function: ARVD is considered an association, rather than the cause of the majority of cases of chronic and end-stage renal failure.10 A haemodynamically significant RAS will lead to a reduction in renal artery perfusion pressure and thus an impairment of renal function simply due to a hydraulic effect, in only a minority of patients. Most commonly, renin and angiotensin levels are increased in the poststenotic kidney, constricting the postglomerular efferent arteriole, which in turn helps to support glomerular capillary hydraulic pressure and filtration rate. This might explain why the use of renin–angiotensin– aldosterone system (RAAS) blocking agents such as angiotensin-converting enzyme (ACE) inhibitors is controversial in the management of ARVD. As glomerular perfusion in these patients is critically dependent upon angiotensin II, the risk of developing acute renal failure is significant, especially if the stenosis is bilateral or affects a solitary functioning kidney. Nonetheless, RAAS-blocking drugs are the only agents proven to slow progression to end-stage renal disease, and acute deterioration in renal function is usually immediately reversible on cessation of treatment.
Cholesterol embolisation may cause acute renal failure in renovascular disease. Patients with severe aortic atheroma undergoing arterial surgical or angiographic procedures, thrombolysis or anticoagulation are at a significant risk of developing renal dysfunction secondary to cholesterol emboli. Associated clinical features include livedo reticularis, proteinuria and eosinophilia. Proteinuria appears to be a key marker of renal damage in patients with ARVD and a prospective study has shown that it can be the main predictor of future deterioration in function.11
Studies in large cohorts of patients with ARVD have shown that there is often poor correlation between the degree of anatomical atheromatous stenosis, glomerular filtration rate (GFR) and overall renal function.12,13 Patients with unilateral ARVD can have GFRs that range from normal to stage 5 kidney disease. Nuclear studies in patients with unilateral stenosis reveal that GFR is the same or even lower in the non-stenotic kidney. This lack of correlation between the severity of renal ischaemic injury and kidney function may explain why renal function often fails to improve significantly after revascularisation despite restoration of renal artery patency.
Hypertension: The pathophysiology of hypertension differs in patients with unilateral and bilateral RAS. In both, a drop in renal perfusion pressure distal to the lesion induces an increase in the activity of the RAAS. Vasoconstriction as well as salt and water retention contribute to the initial rise in systemic blood pressure, which tends to increase perfusion pressure of the poststenotic kidney towards normal. In patients with unilateral disease, perfusion pressure also rises in the contralateral, non-stenotic kidney, inducing a pressure natriuresis response. Salt and water excretion increase in the non-stenotic kidney, promoting a return of extracellular volume towards normal. Initially, therefore, patients may experience marked reductions in blood pressure in response to RAAS blockade.
ARVD and cardiovascular disease
There is a high incidence of adverse cardiovascular events in patients with ARVD as compared with age-matched subjects with normal renal arteries. ARVD is present in 33–44% of patients with peripheral vascular disease and 38% of those found to have an abdominal aortic aneurysm.14,15 In an autopsy series of 346 cases of brain infarcts, RAS was found in 10.4% and carotid artery stenosis in 33.6% of subjects.16
In a large group of patients in whom renal arteriography was performed at the time of cardiac catheterisation, 15% of patients were found to have significant RAS. Those with RAS had a much higher incidence of adverse cardiovascular events as compared with patients without ARVD.17 There was also a direct correlation between the degree of stenosis and overall survival. Patients with > 95% narrowing had only an approximately 40% 4-year survival as compared with 80% in those with normal arteries. These findings were independent of whether or not the patients subsequently underwent revascularisation.18
Sudden-onset left heart failure in patients with no previous cardiac history and well-preserved overall cardiac function (‘flash’ pulmonary oedema) is a manifestation of ARVD. It affects over 10% of patients, especially those with evidence of bilateral RAS.19
Management options
Medical therapy: Optimal medical management should include blood pressure control, antiplatelet therapy, cholesterol management, adequate glycaemic control in diabetics, smoking cessation, diet and exercise (see Chapter 1). It should be noted that the addition of statin therapy to patients with RAS is associated with a reduced likelihood of the progression of the stenosis.20
Antihypertensive therapy: Although antihypertensive therapy is proven to be effective in preventing adverse events in patients with essential hypertension, there are no data on its effects on outcomes in patients with ARVD. A target blood pressure of < 140/90 mmHg is recommended for individuals without other comorbidities, whereas a lower goal of < 130/80 mmHg is recommended for patients with hypertension and diabetes or renal disease with significant proteinuria. Most patients will require combinations of antihypertensive agents to achieve optimal blood pressure control. Surprisingly, RAAS-blocking agents are the first-line antihypertensive choices in patients with ARVD, especially those with evidence of chronic parenchymal disease. Patients should be closely monitored for elevations in serum creatinine and potassium concentrations, particularly in the cases of bilateral RAS or in a solitary kidney.
Endovascular treatment of ARVD: In comparison to surgical techniques, renal stenting is a relatively simple procedure and less hazardous, although careful peri-interventional care is necessary. However, the risk/benefit balance of revascularisation in atherosclerotic RAS is yet to be determined. The recent ASTRAL trial21 appears to cast doubt on the benefits of an endovascular approach (see below). However, it may be prudent to reserve the procedure for patients in certain subgroups who may be at greatest risk. These include the following indications:
• recurrent flash pulmonary oedema;
• dialysis-dependent acute renal failure (in such patients with cortical preservation, there is little to be lost and much to be gained by intervention).
Less certain indications include:
• severe stenosis (> 70%) in a single functioning kidney with deterioration of renal function;
• severe bilateral stenosis (> 70%) with deterioration of renal function;
• ACE inhibitor-related uraemia in patients who require ACE inhibitors for cardiac disease.
Imaging and work-up: Contrast-enhanced MRA had all but replaced other imaging techniques until the association with NSF was recognised. MRA provides the information required prior to renal artery stenting22 (Fig. 15.1a). If a patient has an estimated GFR of < 60 mL/min, then computed tomography angiography (CTA) should be considered (Fig. 15.1b), which carries the alternative, but perhaps more benign, risk of contrast-induced acute kidney injury. If both MRA and CTA are contraindicated, then CO2 angiography can be used.
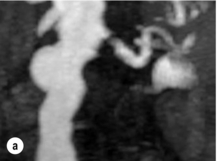
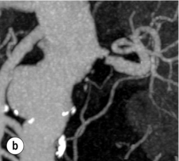
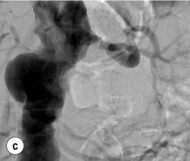
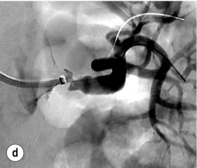
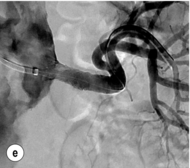
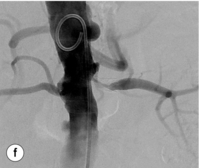
Figure 15.1 (a) Magnetic resonance angiography shows a tight left renal artery stenosis. (b) Corresponding CTA. (c) Conventional catheter angiogram from a brachial approach at the time of treatment confirms these findings. (d) Angiography following cannulation but prior to stent placement. (e) Angiography following stent placement. (f) Angiography demonstrating in-stent stenosis in a different patient.
Renal artery anatomy can be determined from the pre-procedural imaging, allowing placement of the stent to cover the lesion and protrude 1–3 mm into the aorta. Kidneys less than 8 cm in length are generally unsuitable for revascularisation. Patients should be adequately hydrated at least 12 hours before and 12 hours following the procedure with intravenous fluids.23 There is some evidence that administration of N-acetylcysteine reduces the incidence of contrast nephropathy.24 Patients should receive antiplatelet therapy such as aspirin or clopidogrel for life, and be closely followed up for changes in renal function and blood pressure control.
Procedure (Fig. 15.1c–e): Stenting has effectively replaced angioplasty as the first line in endovascular treatment of atherosclerotic RAS (see later).25 Typically via a femoral approach, a 5 F or 6 F shaped sheath is introduced into the abdominal aorta. The renal artery is cannulated and an 0.014–0.018 inch wire is positioned in the renal artery. This allows for usage of low-profile systems for predilatation and stent placement. Vasospasm can be prevented with administration of vasodilators such as glyceryl trinitrate through the sheath. Balloon-expandable stents are now widely used as they are easier to position accurately to achieve full renal ostium coverage.
In patients at risk of contrast-induced nephropathy, alternative contrast agents such as carbon dioxide are available. Cholesterol embolisation may occur at the time of renal artery stenting,26 and some authors advocate the use of embolic protection devices.27,28 None of the present devices are designed for renal intervention and may add to the complexity of the procedure. Data from treatment of other arterial beds have shown a reduction in complications when statins are used, and adminstration prior to RAS should be considered.
Complications: For renal PTA/stenting the literature quotes complication rates ranging from 0% to 66%.29 Major complications include:
• acute deterioration in renal function (usually secondary to contrast acute kidney injury);
• cholesterol embolisation (insidious onset, elevated ESR, eosinophilia, livedo reticularis);
• renal artery perforation (rare) – should this occur, it can usually be treated either by balloon tamponade or a stentgraft.
Results of angioplasty and stenting: The treatment of clinically stable patients with high-grade RAS is highly controversial.
Although ASTRAL showed no benefit in revascularisation, recently Kalra et al. have published prospective cohort data suggesting a benefit of stenting among patients with RAS and chronic kidney disease (CKD).35
Re-stenosis and drug-eluting stents: Re-stenosis can be an issue in renal artery stenting as in other vascular territories (Fig. 15.1f). If it does occur, it will often be seen in the first 3–6 months after the procedure. Figures vary but a re-stenosis rate of 15% at 6 months is commonly seen.35 Although bare-metal stenting is superior to PTA alone, drug-eluting stents hold the promise of reducing re-stenosis rates further.
The GREAT trial37 compared sirolimus-eluting stents with bare-metal stents in a small (n = 106) non-randomised trial. Results showed a trend favouring sirolimus-eluting stents but none of the measures reached statistical significance, possibly due to underpowering.
Surgical treatment: There are a range of surgical options available to treat renal artery disease (Box 15.1). Current guidelines recommend surgery in patients with ARVD who have indications for revascularisation and have multiple small renal arteries or require aortic reconstruction near the renal arteries for other indications (e.g. aneurysm, severe aortoiliac occlusive disease). The site of the lesion is also important, and if extra-anatomical bypass is considered, the condition of the donor visceral vessels must be optimal. In patients with renal artery occlusion, renal biopsy (performed either preoperatively or perioperatively) can indicate whether the kidney is viable and functionally salvageable on the basis of collateral vessels. However, this procedure is not without risk of bleeding and even the loss of a kidney.38 Endarterectomy and bypass grafting are the two main surgical options for revascularisation.
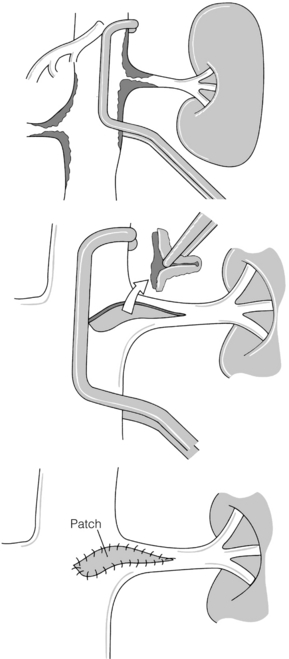
In the presence of aortic aneurysmal disease affecting the renal ostium, aortic graft and renal bypass may be indicated, particularly if the aneurysm is not suited to EVAR. Surgical options include a 6–8 mm limb of Dacron or polytetrafluoroethylene (PTFE) graft sutured onto the aortic graft with an end-to-end renal anastomosis and then bypass on to the affected renal artery in either an end-to-end (usually easiest) or end-to-side manner. Where bilateral RAS is present, an inverted bifurcated Dacron graft is preferred. When the pattern of aneurysm disease dictates that a suprarenal clamp is required for open surgery, transaortic endarterectomy may be performed. The ostial lesion is then carefully endarterectomised and the procedure is completed with patch closure (Fig. 15.2). Five-year patencies in large centres can reach 90%.39
Extra-anatomical bypass grafting is an attractive option for patients with unilateral RAS in the absence of significant aortic disease. Access is obtained via a subcostal incision, without the need for aortic cross-clamping or extensive dissection, and revascularisation is achieved using inflow from either the hepatic or splenic artery (Fig. 15.3a). An interposition saphenous vein graft may be used where there is insufficient arterial calibre and length for an end-to-end anastomosis (Fig. 15.3b). The inferior vena cava, the right renal vein and often the left renal vein must be fully mobilised. On the left side, the splenic artery is dissected from its midpoint from the pancreas. Splenectomy can be avoided as there is a rich collateral supply and perfusion via the short gastric arteries. The Cleveland clinic reports 175 extra-anatomical bypass procedures over a 12-year period,40 with 2.9% operative mortality. Graft patency reached 96%, renal function improved in 40% and hypertension was improved or cured in three-quarters of the series.
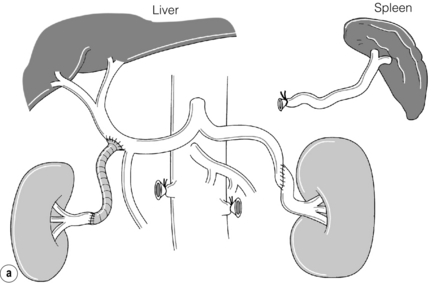
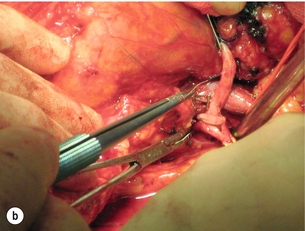
Figure 15.3 (a) Extra-anatomical renal revascularisation from the hepatic or splenic arteries. (b) Hepatorenal bypass using the long saphenous vein.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree