CHAPTER 48 Vascular Physiology
Many cell types make up the walls of blood vessels. Endothelial cells make up the inner layer. This intimal endothelial layer is surrounded by a variable number of layers of smooth muscle cells comprising the medial layer. The adventitial layer surrounds the vascular smooth muscle layers. This last layer is responsible for providing structural integrity to the blood vessel, particularly larger arteries. Initially, the endothelium was thought to serve mainly as a barrier to the diffusion of macromolecules, but recently much has been learned about the pivotal role it plays in vascular function, regulation of vascular tone, and control of local blood flow.1 Smooth muscle cells also control vascular tone via humoral vasoactive factors, neural mediators, or local paracrine factors (Fig. 48-1).
The classification of microvessels based on structural characteristics is rather arbitrary, and there is a lack of uniformity in the definitions of microvascular segments such as small arteries, arterioles, venules, and so on. The transition between these segments is gradual and there is no clear demarcation between them. In general, microvessels are defined as vessels less than 300 μm in internal diameter. Capillaries are the smallest blood vessels, defined as vessels whose walls are composed of only endothelial tubes. The microvessels through which blood flows toward capillaries are arterial microvessels, and those that drain from capillaries are venous microvessels.2 Arterial microvessels usually have three coats—a thin tunica intima; a relatively thick tunica media, composed of one to several layers of smooth muscle cells disposed circumferentially; and a tunica adventitia, made up of fibrous elements and fibroblasts. Venous microvessels collect the blood from capillaries and have thinner vascular walls than arterial microvessels. Venules, 50 μm in diameter, do not possess smooth muscle cell layers. Smaller venules have only endothelial cells and pericytes; because these venules are the most permeable, they play an important role in substance exchange.
The various vascular beds in the body possess many similarities and subtle differences. The regulation of myocardial perfusion depends on many intrinsic and extrinsic factors that may be affected by atherosclerotic lesions. In the coronary circulation, it has been shown that vasomotor regulation of vessels, in addition to the actual anatomy, plays an important role in coronary perfusion and operative decision making. Blood flow is also largely dependent on the resistance generated by the microcirculation. Although early vasomotor regulation studies consisted of indirect assessments using measurements of flow and calculations of resistance, more recent investigations into the properties of the intact coronary circulation have yielded much information, as have modern methods of analysis for interpretation of physiologic data.3–6
VASCULAR RESISTANCE
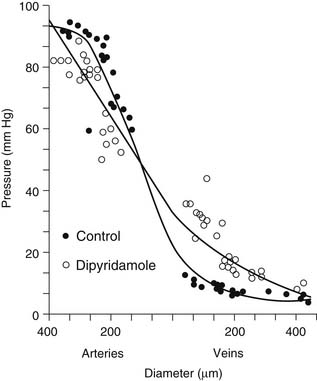
An understanding of vascular resistance is important, as it is these resistance vessels that cause pressure losses and are responsible for regulation of perfusion. Initially, it was thought that the precapillary arterioles were responsible for vascular resistance, with little resistance involvement by the vessels larger than 25 to 50 μm in diameter. Subsequent work revealed that over half of total vascular resistance is caused by vessels larger than 100 μm, and this can be observed in vessels larger than 300 μm.7,8 Also, contrary to previous belief, the venous circulation, under similar conditions of vasodilation, may account for up to 30% of vascular resistance. Figure 48-2 shows that under the vasodilatory effects of dipyridamole, larger arteries and veins assume a greater role in resistance.8,9 Similarly, ischemia results in a significant redistribution of vascular resistance.9 This reveals that the distribution of vascular resistance is dynamic and is dependent on vascular tone, among other factors.
In the coronary circulation, pressure losses are also caused by vessels as they course from the epicardium through the myocardium.10 This is accentuated further in the setting of cardiac hypertrophy. Such a phenomenon is particularly relevant clinically, as it plays a role in explaining the pathophysiology of subendocardial infarcts, as shown in Figure 48-3. The hypertrophied pathologic state causes a decrease in the perfusion pressure of the subendocardium, predisposing it to ischemia and infarction.11
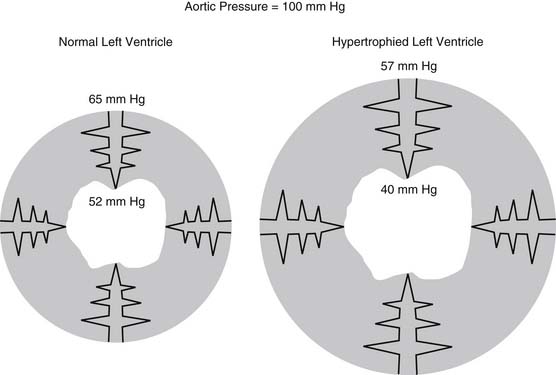
Figure 48–3 Transmural losses of coronary perfusion pressure in normal and hypertrophied hearts. Pressures were measured using micropuncture–servo null techniques in hearts perfused via the left main coronary artery at 100 mm Hg www.lww.com.
(Adapted from Fujii M, Nuno DW, Lamping KG, et al. Circ Res 1992;71:120-6.)
REGULATION OF VASCULAR TONE
Intrinsic and Extrinsic Vasomotor Control
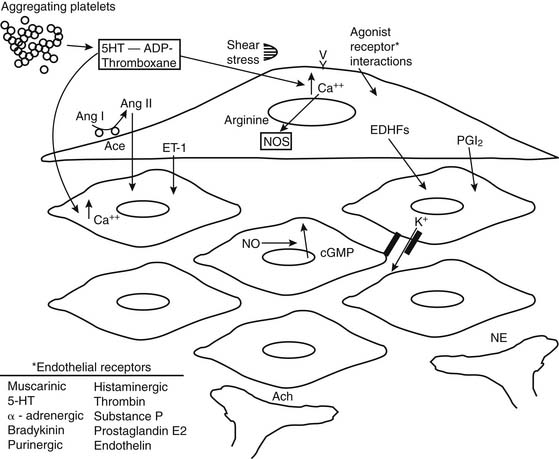
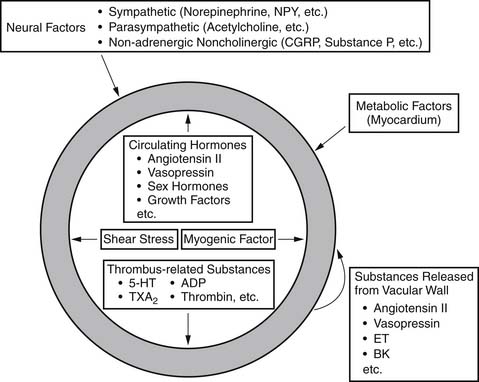
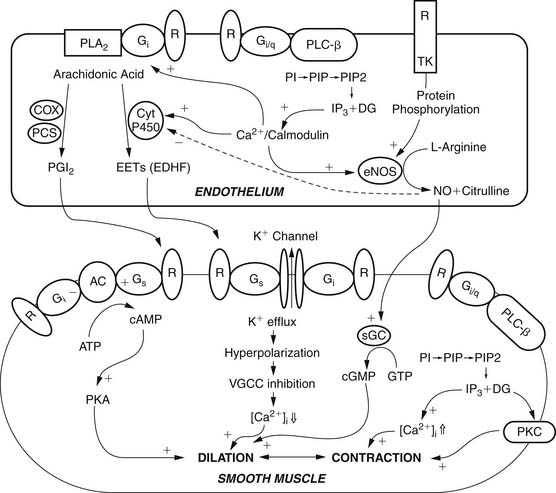
Vessels possess intrinsic control mechanisms for maintaining the homeostasis of the local microenvironment in the face of stress. Vasomotor tone regulation is a complex process that is influenced by the intrinsic properties of the vessel wall, by local innervation, and by substances from surrounding parenchymal tissue. Properties intrinsic to the vessel wall and interactions with adjacent tissues work together to promote metabolic regulation and autoregulation. Endothelial regulation of vasomotor tone is also critically involved in organ perfusion. Vascular responses to endogenous substances are summarized in Figure 48-4. All of these factors play an especially significant role in the setting of microvascular tone.2
On the basis of in vitro observations, Jones and colleagues found that larger arterioles are more sensitive to shear stress than myogenic factors, whereas small microvessels are more sensitive to metabolic factors.12 Based on this they proposed three distinct microdomains that are governed by distinct forms of regulation. They have divided the arterial microvessels into (1) small arterioles (<50 μm), which are most sensitive to metabolic mediators; (2) intermediate arterioles (50 to 80 μm), where myogenic mechanisms predominate; and (3) large arterioles (80 to 150 μm), where flow-induced dilation most potently occurs. This model provides insight for understanding the regulation of microvessels, although there is undoubtedly overlap among these three components.
Jones and colleagues hypothesized that the longitudinal disposition of these three microdomains may enable the integrated adjustment of flow conductance in the face of various influences, such as increased metabolism, a reduction in perfusion pressure, and so on, by affecting other microdomains together.12 For example, the dilation of small arterioles by augmented metabolism produces a decrease in luminal pressure in upstream microvessels, leading to the dilation of intermediate arterioles by decreasing the myogenic tone. These microvascular dilations could produce an increase in shear stress and could result in enhanced flow-induced dilation in large arterioles. As a result, all sizes of arterial microvessels dilate in response to the metabolic stimulation. The marked longitudinal heterogeneity of microvascular responses may be at least partly explained by this microdomain hypothesis.
Role of the Endothelium
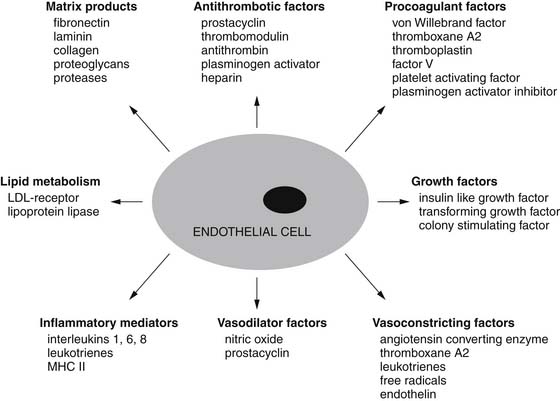
The endothelium plays a pivotal role in vasomotor tone regulation. Many substances can affect tone via endothelium-mediated mechanisms. Endothelial cells also release several substances that affect coronary resistance, such as nitric oxide (NO•), prostaglandins, a hyperpolarizing factor, endothelin, and reactive oxygen species (ROS). These are summarized in Figure 48-5. As the major regulatory molecule, NO is produced by a constitutively expressed enzyme known as endothelial nitric oxide synthase (eNOS or NOS-3). NO is formed as a result of a series of electron transfers from NADPH to the flavins FAD and FMN on the reductase domain, and electron transfer to a prosthetic heme group in the oxygenase domain. When heme reduction occurs, arginine is catalyzed to citrulline and nitric oxide. The NO• formed diffuses to underlying vascular smooth muscle, where its actions include stimulation of soluble guanylate cyclase, increasing the level of cyclic guanosine monophosphate (cGMP) and prompting vasodilation via activation of cGMP-dependent protein kinase.13 Although binding of calcium/calmodulin is a prerequisite for activity of eNOS, other events, such as phosphorylation,14 membrane binding,15 binding of eNOS with heat-shock protein 90, and association with the integral membrane protein caveolin,16 can also modulate NOS activity. Also, NO• may undergo reactions with thiol-containing compounds to form biologically active nitroso molecules.17
Although eNOS is expressed constitutively, it undergoes important gene expression regulation by factors such as shear stress, endothelial cell growth, hypoxia, exposure to oxidized low-density lipoprotein, and exposure to cytokines.18–20 In the coronary circulation, the release of NO• confers a state of basal vasodilation. Hence, administration of NO synthase antagonists produces an increase in resting coronary resistance. On the other hand, when substances such as acetylcholine and bradykinin are administered, coronary microvessels of all sizes dilate. Endothelial NO production is affected by a variety of mechanisms in many disease states. The signal transduction pathways through which NO acts are summarized in Figure 48-6. It is likely that the most important pathway involves activation of soluble guanylate cyclase, which catalyzes the formation of cGMP from guanidine triphosphate. The cGMP serves as an allosteric regulator of the enzyme cGMP-dependent protein kinase (PKG). PKG phosphorylates contractile proteins and ion channels, decreasing intracellular calcium and the sensitivity of contractile proteins to intracellular calcium. The binding of NO to cytochrome oxidase in the mitochondria leads to regulation of oxygen consumption and in turn may affect oxygen demand. Similarly, the receptors of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are also particulate forms of guanylate cyclases, and these substances produce vasodilation via similar pathways. NO is also released in response to sodium nitroprusside and organic nitrates.
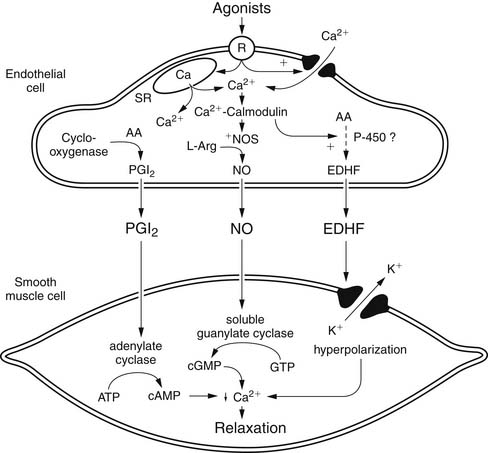
Although NO is the major regulator of vascular tone, there are other factors that modulate endothelium-dependent vascular tone in coronary, pulmonary, and peripheral circulations. Endothelium-derived hyperpolarizing factor (EDHF) is an example. The endothelium-dependent hyperpolarization of vascular smooth muscle is mediated by the opening of a calcium-dependent potassium channel or by activating Na/K-ATPase. The role of the various EDHFs probably varies depending on the vessel size, the species, and the vascular bed under consideration. When the vascular smooth muscle is hyperpolarized, voltage-sensitive calcium channels are closed, leading to a reduction in intracellular calcium. Several different EDHFs exist, such as epoxyeicosatrienoic acid (EET), a cytochrome p450 metabolite of arachidonic acid. Other possible EDHFs include potassium and hydrogen peroxide. Prostaglandin synthesis by the endothelium also contributes to modulation of tone in the microcirculation. The predominant prostaglandin produced by endothelial cells is prostacyclin (PGI2). There is substantial interaction between nitric oxide, EDHF, and prostacyclin. A major stimulus for release of prostacyclin, NO, and EDHF is shear stress, or the tangential force of fluid as it flows over the endothelium, resulting in flow-dependent vasodilation. Interestingly, the importance of nitric oxide seems to decline and the role of the EDHF increases as blood vessels decrease in size. Consequently, the production of EDHF may increase when nitric oxide is low. The interaction of endothelial cells with vascular smooth muscle cells and the intermediates involved is outlined in Figure 48-7.
Role of Metabolism and Autoregulation
The ability of a vascular bed to adjust its tone to maintain a constant flow during changes in perfusion pressure is termed autoregulation.21 This process is most effective in the coronary circulation when pressure is between 40 and 160 mm Hg. The subendocardium and the subepicardium differ in the range of pressures over which autoregulation can be observed: in the subendocardium, flow begins to decrease at pressures of less than 70 to 75 mm Hg, whereas this happens at significantly lower pressures in the superficial layers of the myocardium.22 Clinically, systemic arterial hypertension affects the range over which autoregulation occurs in the subendocardium, such that flow begins to decline at even higher pressures. Such a change in subendocardial perfusion pressure in the setting of hypertrophic myocardium increases the likelihood of subendocardial ischemia. Also, patients with systemic hypertension may have an increased lower limit of autoregulation and thus may suffer brain ischemia during surgery, or at other times even at pressures sufficient to sustain adequate perfusion in patients with normal blood pressure.
Flow-Induced Dilation
Flow-induced dilation is a ubiquitous phenomenon of blood vessels in various organs and animals, including humans.2,23,24 Flow-induced dilation plays important physiologic roles in the following ways: (1) it protects the vessel wall against friction induced injury, (2) it prevents the vascular steal phenomenon by dilating upstream vessels when there is focal hyperemia, (3) it reduces the heterogeneity of flow distribution, and (4) it buffers the pressure distribution if there are rapid pressure changes.
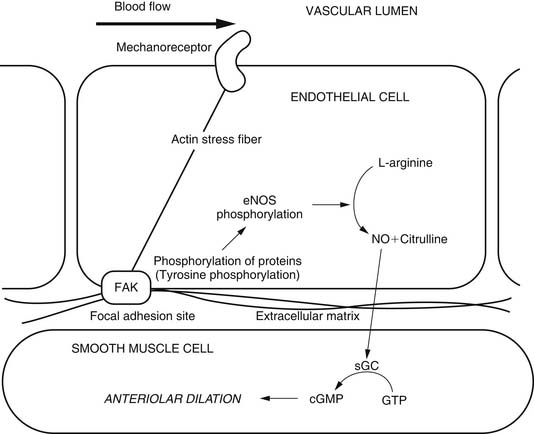
Flow is sensed by endothelial cells through as yet unidentified mechanoreceptors. In contrast to the myogenic response, the endothelium is required for flow-induced dilation. Regulation of flow-mediated vasodilation is controversial, and both NO and prostaglandins have been shown to be involved, at least in porcine coronary arterioles.25,26 The exact mechanism of regulation may depend on age, vessel size, and the vascular bed under consideration. A possible flow-induced arteriolar dilation mechanism is summarized in Figure 48-8.
Autoregulation is mediated by the actions of several factors such as NO, EDHF, and adenosine. Removal of a particular factor does not prevent autoregulation, as the other factors seem to take over its function. Adenosine and hydrogen peroxide also cause hyperpolarization of vascular smooth muscle. From the work of Duncker and colleagues, and others, we now know that several factors work together to influence metabolic regulation and autoregulation, leading to adequate regulation of coronary vascular tone despite interruption of any particular pathway.21
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree