Vascular interventions of the mesenteric vessels are typically performed to treat intestinal ischemia. Although acute mesenteric ischemia (AMI) and chronic mesenteric ischemia (CMI) differ in their clinical presentation, etiology, and management, both are a result of inadequate perfusion of the mesenteric vessels that can ultimately result in bowel infarction. It is essential that the treating vascular interventionalist should have a thorough knowledge of the current imaging modalities and endovascular therapies utilized in the diagnosis and management of mesenteric ischemia, respectively. This chapter will focus on the relevant anatomy, diagnostic evaluation, interventional techniques, and current research pertaining to mesenteric ischemia.
ANATOMIC CONSIDERATIONS
The small and large intestines are supplied by branches from three major arteries arising from the anterior surface of the abdominal aorta: the celiac trunk, the superior mesenteric artery (SMA), and the inferior mesenteric artery (IMA). Corresponding to their embryologic origins, the celiac trunk supplies the foregut region between the esophagus and the distal duodenum, the SMA supplies the midgut area between the proximal jejunum of the small intestine and the midtransverse colon, and the IMA supplies the hindgut from the midtransverse colon to the rectum (1). Within and across the vascular distributions, there is wide variability in normal individuals.
Arising at the level of T12, the celiac trunk is the first major branch of the abdominal aorta. In more than two thirds of cases, it courses anteriorly for 1 to 2 cm and bifurcates into the splenic and common hepatic arteries before giving rise to the left gastric artery (Figs. 23.1). In approximately one third of patients, the celiac trunk trifurcates into three main branches. In a very small percentage of cases, the origin of the celiac artery fuses with the SMA, creating a celiacomesenteric trunk (Figs. 23.2).
Typically, the splenic artery arises within 2 cm of the celiac trunk and has a torturous leftward and posterior course toward the hilum of the spleen. The first branch of the splenic artery is the dorsal pancreatic artery, which, as the name implies, supplies the posterior aspect of the pancreas and gives rise to the transverse pancreatic artery that perfuses the pancreatic body and tail. The dorsal pancreatic artery originates from the splenic artery in 39% of adults, but in the remaining 61% originates from another vessel such as the right hepatic artery, SMA, or celiac trunk (2). The distal splenic artery provides multiple short gastric arteries and the left gastroepiploic artery, which supply regions of the stomach and the greater omentum.
The common hepatic artery courses rightward and anteriorly, dividing into the proper hepatic artery and the gastroduodenal artery (Figs. 23.3). The proper hepatic artery subsequently divides into the right and left hepatic arteries; however, in 19% of patients, the right hepatic artery originates from the SMA and is referred to as a replaced right hepatic artery. Similarly, in less than 2% of cases, the entire common hepatic artery may arise from the SMA and is named a replaced common hepatic artery. In addition, the gastroduodenal artery, which provides branches to the stomach, pancreas, and proximal duodenum, arises from the common hepatic artery in 75% of patients but may originate from the left hepatic artery (11%) or right hepatic artery (8%) (3).
The SMA typically arises at the level of L1 and originates from the aorta approximately 1 cm inferior to the celiac trunk and superior to the renal arteries (Figs. 23.4). Its origin is posterior to the pancreas and splenic vein and the artery courses anteriorly toward the third portion of the duodenum. The SMA lies to the left of the superior mesenteric vein (SMV) as it crosses over the third portion of the duodenum and is usually posterior to the SMV as it enters the mesentery.
The SMA supplies several individual branches that arise sequentially from the right side of the main trunk including the inferior pancreaticoduodenal, middle colic, right colic, and ileocolic, and multiple jejunal (4–6) and ileal (8–12) branches that arise from the left side of the main trunk. The inferior pancreaticoduodenal artery, which anastomoses with branches from the gastroduodenal artery, may arise either as two separate branches or as a common single trunk. In succession, the middle colic artery and the right colic artery arise from the SMA and supply blood to the transverse and ascending colon, respectively. The right colic artery originates as a branch of the middle colic artery in 52% of cases (3). The ileocolic artery is the most constant branch of the SMA and can be used as an important landmark for angiographic interpretation. It provides collateral vessels to the terminal ileum, cecum, and first half of the right colon. Aberrant arteries arising from the SMA are a relatively common finding and include the hepatic artery, right hepatic artery, cystic artery, splenic artery, gastroduodenal artery, and left gastric artery (4).
The IMA typically arises at the level of L3 and is significantly smaller than either the celiac trunk or SMA (Figs. 23.5). The IMA courses to the left and provides branches to the distal transverse, descending, and sigmoid colon before it terminates by dividing into two superior rectal (or hemorrhoidal) arteries. It gives origin to several branches from its left side, including the left colic artery, colosigmoid artery, and rectosigmoid artery. The left colic artery, the first and major branch of the IMA, travels adjacent to the inferior mesenteric vein (IMV) and bifurcates at the splenic flexure.
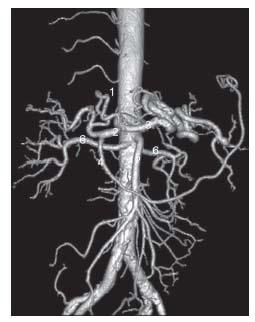
Figure 23.1 • CT angiogram of normal mesenteric arterial anatomy. Surface shaded reconstruction demonstrates typical anatomy. (1) Left gastric artery. (2) Common hepatic artery. (3) Splenic artery. (4) Gastroduodenal artery. (5) Superior mesenteric artery. (6) Renal arteries.
Collateral connections between the mesenteric vessels are well recognized and are essential for maintaining adequate perfusion when major mesenteric vessels are occluded or surgically ligated. An understanding of the collateral circulation is mandatory when performing endovascular revascularization procedures. Between the SMA and IMA, the major collateral arcade consists of the marginal artery of Drummond and meandering artery of Moskowitz, composed of branches of the ileocolic and right, middle, and left colic arteries (Figs. 23.6). In the absence of arterial occlusion, the marginal artery of Drummond may not be clearly visible on angiography, but it can enlarge significantly when the SMA or IMA is occluded. The arc of Riolan refers to a set of collaterals that communicates between the middle colic and left colic arteries at the splenic flexure. If the meandering artery of Moskowitz within this arc is visible on angiography, it is a strong indication that the SMA or IMA is occluded (Figs. 23.7) (5). The collateral circulation between celiac axis and SMA is predominantly via the pancreaticoduodenal arcade (Figs. 23.8). In some patients, persistence of a direct celiac and SMA communication may exist and is termed the arc of Buehler (Figs. 23.9).
In the mesenteric venous circulation, the SMV is typically a single vessel formed by a right and a left branch that receive blood from several veins (i.e., ileocolic, right colic, and middle colic veins). In other patients, the right and left branches may not form a single SMV, but instead join the splenic vein separately to form the portal vein. Similarly, the IMV may drain into the splenic vein or into the SMV after receiving blood from its mesenteric tributaries.
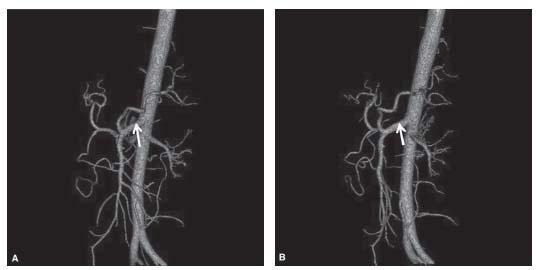
Figure 23.2 • Celiacomesenteric trunk. A,B: Shaded surface CTA reconstructions demonstrate celiacomesenteric trunk (arrow). C: Conventional angiographic image shows celiacomesenteric trunk.
ACUTE MESENTERIC ISCHEMIA
AMI is a potentially fatal vascular emergency that necessitates early diagnosis and intervention to restore mesenteric blood flow and prevent bowel necrosis. Despite the development of modern treatment modalities, AMI remains a diagnostic challenge for clinicians due to its non-specific clinical presentation. The delay in diagnosis contributes to the high incidence of bowel infarction and the continued high mortality rate of 60% to 80% associated with this diagnosis (6).
The key to diagnosis of AMI is maintaining a high degree of clinical suspicion in high-risk individuals (see Table 23.1). Imaging may or may not be helpful. The utility of plain radiographs is limited since findings of mesenteric thickening and thumbprinting are nonspecific and relatively late manifestations of the condition. The more specific findings of portal venous gas and intramural pneumatosis typically occur with bowel infarction and are associated with a poor prognosis. In addition, other noninvasive imaging modalities such as magnetic resonance angiography (MRA) and duplex ultrasound generally do not play an important role in the diagnostic workup of AMI because of its acute presentation. However, recent studies have shown that computed tomography angiography (CTA) has provided a noninvasive alternative to conventional angiography for the diagnosis of AMI (7–9). Multidetector CT scanners particularly with sagittal reformatting are capable of demonstrating the proximal mesenteric vessels very well. In one prospective study involving 62 patients undergoing biphasic multidetector row CT, AMI was identified as a possible diagnosis in all 26 who were ultimately determined to have mesenteric ischemia. Notably, only eight of these patients had arterial abnormalities seen on the CT angiogram, emphasizing the importance of associated bowel findings in making the diagnosis (7). CT findings of bowel wall thickening, intramural or portal venous gas, solid organ infarction, or lack of enhancement of bowel wall all aid in the diagnosis of mesenteric ischemia. However, it is important to recognize the limitations of CTA, as distal branches are not reliably evaluated compared with conventional angiography, and CTA is rarely able to diagnose nonocclusive mesenteric ischemia (NOMI).
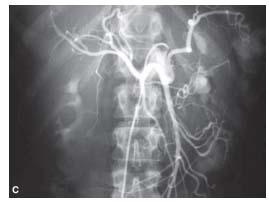
Figure 23.3 • Anatomy of the celiac axis. Selective angiogram of celiac trunk shows conventional celiac anatomy. (1) Left gastric artery. (2) Common hepatic artery. (3) Splenic artery. (4) Gastroduodenal artery.
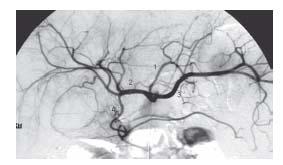
Figure 23.4 • Anatomy of the superior mesenteric artery. Selective angiogram of SMA shows conventional anatomy. (1) Ileocolic artery. (2) Right colic artery. (3) Jejunal branches. (4) Ileal branches.
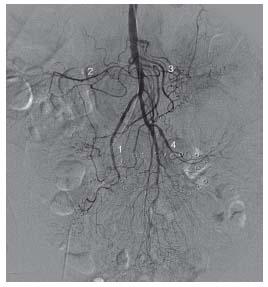
Figure 23.5 • Anatomy of the inferior mesenteric artery. Conventional inferior mesenteric angiogram showing perfusion of descending and sigmoid colon. (1) Left colic. (2) Sigmoid branches. (3) Superior rectal branch.
Despite the recent interest in CTA, the gold standard for the diagnosis of AMI remains digital subtraction angiography. Because early diagnosis and treatment is critically important in AMI to avoid bowel infarction, angiography can provide rapid diagnostic information not available from CT and enables concurrent therapeutic treatment. Angiography begins with biplane abdominal aortography. While the anteroposterior view is best for demonstrating the distal mesenteric blood supply and collaterals, the lateral view is better for visualizing the origins of the major visceral arteries.
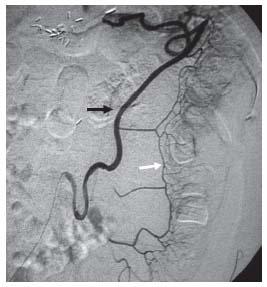
Figure 23.6 • Inferior mesenteric angiogram showing the meandering artery composed of ascending left colic branches (black arrow) and the marginal artery (white arrow).
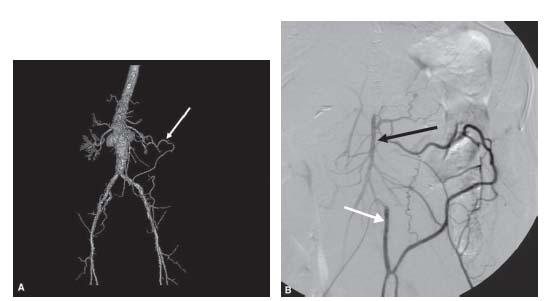
Figure 23.7 • Hypertrophy of meandering artery of Moskowitz in patient with celiac and superior mesenteric artery occlusion. A: Shaded surface CT angiogram shows enlarged meandering artery (white arrow). B: Conventional selective inferior mesenteric artery (IMA) angiogram shows reconstitution of superior mesenteric artery (black arrow) via collateral from the IMA (white arrow). C: Delayed image from selective IMA angiogram shows reconstitution of the celiac axis (black arrow).
Causes and Angiographic Appearance of Acute Mesenteric Ischemia
Although there are numerous causes of AMI (see Table 23.2), the four major etiologies are SMA embolism, NOMI, SMA thrombosis, and mesenteric venous thrombosis (MVT). By far, the most common cause of AMI is embolic occlusion of the SMA (Figs. 23.10), which accounts for 40% to 50% of all cases (10). Emboli are usually cardiac in origin and are associated with mural thrombi from cardiac hypokinesia, valvular vegetations, or arrhythmias in high-risk patients. While 15% of arterial emboli occur at the origin of the SMA, the majority lodge distal to the origin of the middle colic artery, which is the first major branch of the SMA (11). Due to the lack of collateral circulation in the setting of acute SMA obstruction, the onset of symptoms typically manifests as severe abdominal pain, fever, emesis, and diarrhea (sometimes bloody). The classic presentation is significant abdominal pain that is out of proportion to the patient’s physical findings. However, it is not uncommon for a patient to present with an acute abdomen, including peritoneal signs and hypotension secondary to bowel infarction. Due to excessive fluid loss, laboratory findings include metabolic acidosis with elevated lactate levels, leukocytosis, and hemoconcentration. Angiographic findings of an acute embolus include the presence of a filling defect outlined by contrast, occlusion within a convex meniscus, and occlusion more than 3 cm distal to the origin of the SMA (12). In contrast, SMA thrombosis most commonly occurs in the setting of pre-existing stenotic disease at the origin of the vessel and usually spares distal arteries.
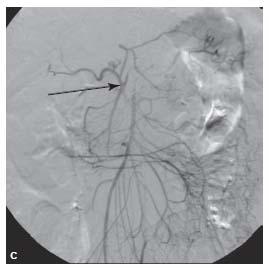
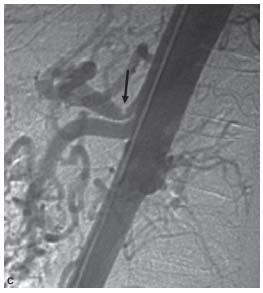
Figure 23.8 • Hypertrophy of pancreaticoduodenal arcade in patient with celiac axis occlusive disease. A: Shaded surface reconstruction CTA demonstrates hypertrophy of pancreaticoduodenal arcade (within white circle). B: Conventional superior mesenteric artery (interrupted white arrow) angiogram showing retrograde flow through the pancreaticoduodenal arcade (within white circle) reconstituting common (white arrow) and proper (black arrow) hepatic arteries. C: Lateral aortogram showing high-grade celiac stenosis (black arrow) in this patient.
NOMI accounts for 20% to 30% of all cases of AMI with a mortality rate of approximately 50% (13). The pathogenesis of NOMI is not clearly understood but involves a period of systemic hypotension associated with diffuse mesenteric vasoconstriction. In low-flow states, peripheral vasodilation may occur with shunting of blood away from the gut. This splanchnic vasoconstriction ultimately causes intestinal hypoxia and necrosis. Congestive heart failure, aortic insufficiency, hepatorenal disease, and vasoactive drugs such as cocaine, digitalis, and dopamine have all been shown to predispose patients to developing NOMI. Unlike patients with SMA embolism who have an acute onset of symptoms, those with NOMI typically have a more protracted clinical course. Because this condition frequently affects critically ill patients, some are unable to describe symptoms and instead undergo unexplained worsening in their clinical condition. More healthy patients may describe nonspecific abdominal pain. Angiographic findings of NOMI include diffuse arterial vasospasm, diminished arterial flow with reflux of contrast from the SMA into the aorta, and narrowing of peripheral mesenteric vessels or a pattern of alternating dilatation and narrowing (12).
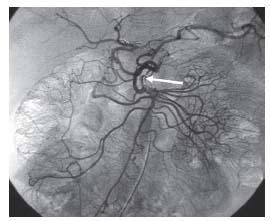
Figure 23.9 • Arc of Buehler. Conventional superior mesenteric angiogram shows communication between the superior mesenteric artery and celiac trunk via the arc of Buehler (white arrow).
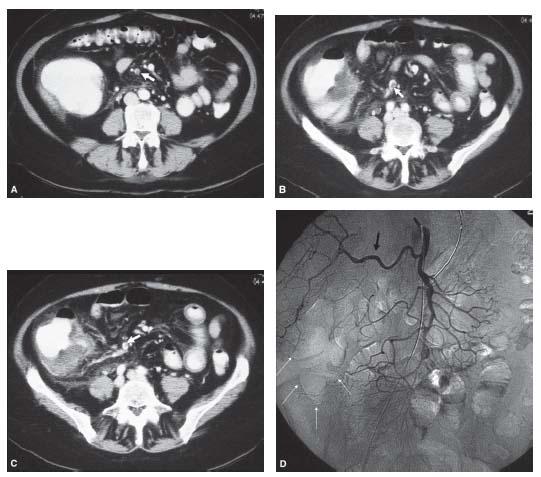
Figure 23.10 • Embolus to the superior mesenteric artery causing bowel ischemia. A–C: Axial enhanced CT images show marked bowel wall thickening of the cecum and distal small bowel and a round filling defect representing an embolus in the ileocolic branch of the superior mesenteric artery (white arrow). D: Conventional superior mesenteric angiogram demonstrates no evidence of filling of the ileocolic branch with complete lack of perfusion to the cecum (white arrows). Note replaced right hepatic artery (black arrow).
Acute mesenteric thrombosis accounts for approximately 25% of all cases of AMI and occurs almost exclusively in the setting of severe atherosclerotic disease (14). Although it can present acutely like a SMA embolism, most patients with SMA arterial thrombosis present with insidious symptoms of postprandial abdominal pain, nausea, and weight loss. Often patients with this condition can tolerate major mesenteric artery obstruction because the progressive nature of atherosclerosis allows the development of important collaterals. Reflecting the more chronic nature of the obstruction, angiographic findings reveal collateral vessels reconstituting distal SMA branches in many cases. The SMA is typically occluded proximally within 1 to 2 cm of its origin but reconstitutes distally via collateral circulation (Figs. 23.11) (12).
Usually located within the SMV, MVT accounts for less than 5% of cases of AMI (Figs. 23.12) (14). In the setting of acute venous thrombosis without adequate collateral formation, intestinal mucosa edema develops and inhibits mesenteric arterial perfusion. Cases of MVT are commonly associated with hypercoagulable states, portal hypertension, oral contraceptives, and recent abdominal surgery (15). Except in the most severe cases, patients with MVT typically present late with nonspecific abdominal pain associated with anorexia, fever, and diarrhea. The angiographic findings of MVT include increased arterial resistance to flow, prolonged and intense mucosal staining of the bowel wall, vasospasm of the mesenteric arteries with persistence of the arterial phase, and lack of opacification of the mesenteric veins or portal vein (12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree