The last two decades have seen a dramatic increase in the number of endovascular procedures performed, due to expansion to treatment in all vascular territories and the use of novel techniques and devices (1). Vascular complications are encountered in 5% to 6% of endovascular procedures and result in significant morbidity and mortality (2). As a result, knowledge of these complications and the ability to anticipate and manage them are crucial for any endovascular specialist. In this chapter, we will review in detail the most commonly encountered access site complications, and briefly discuss other complications common to most endovascular procedures including distal embolization, dissections, perforations, and retrieval of foreign bodies.
ACCESS SITE COMPLICATIONS
Vascular access site complications are some of the most common complications encountered during endovascular procedures. These include:
- Bleeding
- Pseudoaneurysm formation
- Arteriovenous fistula (AVF) formation
Bleeding
The femoral artery is most commonly accessed artery during endovascular procedures (3). Major bleeding complications related to femoral arterial access occur in 1% to 2% of cases and are more common than with brachial and radial artery access. Obesity (body mass index >25), female sex, use of glycoprotein (GP) IIb/IIIa inhibitors, hypertension, chronic kidney disease, and diabetes are the most consistent predictors of localized bleeding (4). High femoral artery puncture along with numerous procedural factors such as performance of interventions, duration of procedure, sheath size, and concomitant venous access have also been identified as predictors of hemorrhagic complications in numerous studies (5).
The most serious bleeding complication is that of retroperitoneal hemorrhage or hematoma (RPH), and has been reported in 0.5% of patients following endovascular procedures. Puncture of the femoral artery above the middle third of the femoral head and insertion of the sheath above the inguinal ligament is associated with RPH. In half of the cases of RPH, the puncture site of the common femoral artery is below the level of the inguinal ligament. RPH in such cases is related to the puncture of the posterior arterial wall, the inferior epigastric artery, and/or multiple access attempts with passage of blood along the facial planes into the superiorly located retroperitoneal space.
The initial symptoms and signs of RPH are nonspecific and may include back, abdominal, or groin pain and lower quadrant tenderness ipsilateral to the femoral puncture site (6). In nearly 50% of patients, the diagnosis is made after significant blood loss has occurred leading to hemodynamic compromise and hypovolemic shock, manifest by tachycardia and hypotension. Almost 75% of cases present within 3 hours of the procedure and thus hypotension during this interval following an endovascular procedure should prompt a compulsive search for a retroperitoneal source of bleeding (7). It is imperative to understand that the diagnosis of RPH is a clinical one, and that when suspected on the basis of the clinical symptoms, and signs, appropriate management should be instituted immediately. This includes the following measures:
a. Apply pressure at and slightly proximal to the femoral puncture site.
b. Stop any anticoagulant or antiplatelet infusions.
c. Obtain robust IV access (minimum of two 16- to 18-Fr. peripheral IV access sites or central venous access) and administering wide-open IV normal saline (i.e., two separate 1-L normal saline bags wrapped in pressure bags).
d. Send blood for emergency type and cross of 3 to 6 units of blood based on the clinical circumstance.
e. Consider the need for platelet infusion if the patient has received abciximab (non-competitive GP IIb/IIIa inhibitor) during the procedure (Table 25.2).
f. Consider the need for reversal of any residual anticoagulant effect of IV heparin using protamine (Table 25.2).
g. If these measures do not result in prompt resuscitation of the patient, localization of the exact site of bleeding by selective iliac artery angiography from upper extremity or contralateral femoral access should be performed (8). If there is evidence of ongoing bleeding, slightly oversized balloon can be used to occlude the source of bleeding. Failed balloon tamponade can be rescued by administration of thrombin, placement of a covered stent graft, or referral for an open surgical repair. Surgical intervention is required in only 12% of cases and is associated with significant morbidity (7).
Imaging studies may be used to confirm the diagnosis and assess the anatomic extent of the RPH, but only following stabilization of the patient (Fig. 25.1). Nearly all patients require transfusion of blood products and have an extended hospital stay associated with increased costs. RPH is a strong predictor of in-hospital and long-term mortality (9). Mortality from RPH occurs in ~4% of patients. Associated complications of RPH i nclude femoral nerve neuropathy, which occurs in up to 24% to 54% of patients, and deep vein thrombosis secondary to inferior venous cava compression, which occurs in 2% to 10% of cases (10).
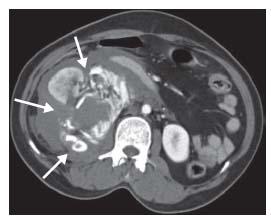
Figure 25.1 • Retroperitoneal hematoma. A large right-sided retroperitoneal hematoma is indicated by the white arrows.
Given the morbidity and mortality associated with RPH, it is important to be aware of measures that prevent its occurrence and bleeding complications in general. These include the use of micropuncture needles to gain arterial access and the performance of femoral access using fluoroscopic landmarking and/or under ultrasound (US) guidance. Additional measures include the use of weight-adjusted unfractionated heparin (or bivalirudin in appropriate patients), early removal of arterial sheaths, avoidance of concomitant femoral venous access where possible, and meticulous post-procedural care. Evidence regarding the use of access-closure devices and the incidence of hemorrhagic complications following percutaneous coronary intervention has been discrepant, which likely relates to differences in patient populations, variation in closure devices, and the absence of randomized data. For endovascular intervention, the authors’ bias is to avoid closure devices due to a clinical impression of higher rates of ischemic complications with these devices in the patient population with peripheral artery disease.
Pseudoaneurysm Formation
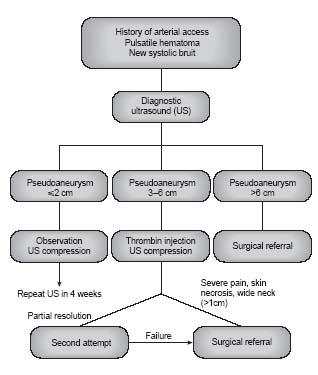
The incidence of pseudoaneurysms after femoral access for endovascular procedures is estimated to be between 0.6% and 6%. Larger catheters, anticoagulant use, and simultaneous catheterization of the femoral artery and vein are predictors of pseudoaneurysm formation. Some studies have indicated that puncture of the superficial femoral artery (SFA) may lead to a higher risk of pseudoaneurysm formation than with puncture of the common femoral artery (11). The most common clinical scenario in which a pseudoaneurysm is diagnosed is in a patient who has some evidence of post-procedural bleeding, followed by marked pain and swelling at the groin site. Clinical examination reveals a new systolic bruit. Additional complications may occur as a result of compression by the pseudoaneurysm of the adjacent common femoral nerve (nerve palsy) and/or common femoral vein (deep venous thrombosis). Compartment syndrome of the lower extremity and distal embolization of clot formed in the sac of the pseudoaneurysm that migrates through the neck of the pseudoaneursym to the distal circulation are very rare complications. Prompt recognition and management is the key to prevention of significant morbidity (12). Pseudoaneurysms are best-visualized and diagnosed using US and adjunctive Doppler and color Doppler assessment. An echogenic mass, with systolic flow signals at the neck of the sac alongside the femoral artery is diagnostic.
The management of femoral artery pseudoaneurysms is largely driven by the diameter measurement of the aneurysm by ultrasound (Fig. 25.2). Small pseudoaneurysms (i.e., 2 cm) will often resolve with conservative treatment (13). Larger pseudoaneurysms (i.e., >2 cm) usually require intervention. Although prompt surgical repair was formerly the mainstay of pseudoaneurysm treatment, this has been largely replaced by US-guided compression of the pseudoaneurysm neck and/ or by thrombin injection (13). The neck of the pseudoaneurysm is located and mechanical pressure is applied at that site using the US transducer for ~20 minutes initially. This should be performed under conscious sedation because of the severe pain associated with this maneuver. Subsequent compression is often required to assure complete cessation of flow, detected with color Doppler, in the pseudoaneurysm neck. A follow-up US examination is suggested in 24 hours to confirm a durable result. Success rates of around 86% have been reported in patients on anticoagulants.
Thrombin injection into the pseudoaneurysm sac under US guidance is often used as part of an initial strategy with US-guided compression of the neck or following a failed attempt to occlude the pseudoaneurysm neck with manual compression alone (14). Approximately 300 IU of thrombin is injected slowly into the pseudoaneurysm sac for over 10 to 15 minutes. The injecting needle is directed to the base of the sac, away from the neck of the pseudoaneurysm. Cessation of Doppler flow within the pseudoaneurysm signals successful treatment. Post-procedure bed rest is advised. If residual flow is detected, additional doses of thrombin may be injected. A follow-up US examination after successful treatment is often performed at 24 hours and again in 1 week. Excess or rapid injection of thrombin may result in parent vessel thrombosis and distal embolization. During thrombin injection of multiloculated pseudoaneurysms or those with neck diameters >5mm, balloon occlusion of the parent artery during thrombin injection is advised to prevent distal embolization. The success rate for thrombin injection in the treatment of femoral pseudoaneurysms is >96%. In extreme situations, coil embolization of the pseudoaneurysm sac and exclusion of the pseudoaneurysm neck with a stent graft can also be employed. When nonsurgical measures are unsuccessful or are contraindicated (e.g., evidence of infection in pseudoaneurysm), surgical pseudoaneurysm repair should be performed (Table 25.1).
Complications related to nonsurgical treatment of femoral pseudoaneuryms include acute enlargement or rupture of the pseudoaneurysm, vasovagal syncope (most commonly related to pain), and deep vein thrombosis. Distal embolization may occur in about 4% of cases using this strategy and may be related to escape of thrombin from the pseudoaneurysm sac or embolism of preformed thrombus within the sac. The treatment of distal embolization associated with thrombin injection of a femoral artery pseudoaneurysm is managed according to the clinical situation, but will generally require emergent angiography (using contralateral retrograde femoral or ipsilateral antegrade femoral access), followed by mechanical thrombectomy and/or intra-arterial thrombolysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


