Intracranial atherosclerosis is a major cause of stroke and accounts for 8% to 10% of ischemic stroke in mixed patient populations (1–4). The vessels involved are mainly those of the circle of Willis. In addition to atherosclerosis, consideration should be given to other etiologies of intracranial disease such as vasculitis, dissection, embolism undergoing recannulization, moyamoya arteriopathy, postradiation arteriopathy, and infectious vasculitides (5).
The Warfarin-Aspirin for Symptomatic Intracranial Disease (WASID) trial demonstrated that a significant proportion of patients with significant symptomatic intracranial disease (defined as stenosis between 50% and 99%) who are treated medically will experience a recurrent ischemic stroke in the distribution of the stenotic artery (2,6). This is likely related to hypoperfusion from the residual flow-limiting stenosis that is not responsive to antithrombotic therapy. In such patients, restoration of adequate cerebral blood flow should be the primary goal of intervention. Extracranial-to-intracranial bypass surgery has been essentially abandoned due to near doubling of the risk of stroke in patients receiving bypass for severe middle cerebral artery (MCA) stenosis compared with medical therapy (7). With developments in stent technology, endovascular approaches have emerged as feasible and potentially highly effective therapy for those patients who fail medical treatment.
PREOPERATIVE PATIENT SELECTION
The most crucial indication for intracranial stenting is the presence of a symptomatic intracranial atherosclerotic stenosis despite optimal medical therapy. Results from the WASID trial show that in patients with a high-grade stenosis (>70%), the risk for subsequent stroke in the territory of the stenotic artery is 23% at 1 year and 25% at 2 years (6,8,9). Although patients with stenoses of less than 70% are also at increased risk of stroke, the risk is relatively low compared with the risk of endovascular intervention; therefore, patients with greater than 70% symptomatic intracranial stenoses are the most likely to benefit from invasive treatment strategies. In addition, patients must be able to tolerate dual antiplatelet therapy for 30 days or longer and their symptoms should be attributable to the territory distal to the stenotic segment. This last point is critical since the basilar artery (BA) and MCA have many important perforators that originate from their main trunks that often cause clinical syndromes due to parent vessel atherosclerosis. Angioplasty or stenting in such circumstances (i.e., recurrent perforator ischemia) has a high probability of causing perforator occlusion and stroke (10).
ENDOVASCULAR APPROACH
The endovascular approach for intracranial angioplasty and stenting is similar to that of acute stroke intervention, but pretreatment with dual antiplatelet agents is critical. A femoral approach is preferred especially for MCA and internal carotid artery (ICA) procedures. Heparin is given to achieve an activated clotting time (ACT) between 250 and 300 seconds. Treatment for vasospasm should be considered during the procedure although there are no data to support this practice. In defense of this practice, cerebral vessels are particularly prone to spasm, and since proper stent sizing is essential, antispasm treatment may help improve the accuracy of device sizing. A long sheath (6- to 8-Fr. 80-cm Shuttle™ [Cook Medical Inc.]) should be advanced into the common carotid artery (CCA) or subclavian artery (except in the rare patients with no tortuosity and a relatively proximal stenosis where a short sheath may be used) and a 6-Fr. guide (Envoy™ [Cordis Inc.] or Neuron™ [Penumbra Inc.]) should be placed distally in the cervical ICA or vertebral artery (VA). The lesion should then be crossed with a hydrophilic, soft microwire with an atraumatic tip (e.g., Synchro™ or Transcend Floppy™ [Boston Scientific Inc.], among others). The tip of the guide-wire should be positioned distal to the stenosis with great care to avoid placing the wire in small branches or perforators. For terminal ICA and MCA treatment, the wire should be passed into the second or proximal third-order branches of the MCA (Fig. 13B.1A). In the posterior circulation, the wire should be placed in a posterior cerebral artery (PCA) if possible (Fig. 13B.1B). The authors’ approach is to always predilate the lesion with an undersized, over-the-wire balloon (e.g., Maverick™ or Gateway™ [Boston Scientific Inc.], among others) keeping in mind that vessel rupture or dissection with subarachnoid hemorrhage are often fatal in this setting (Fig. 13B.1C). This practice permits adequate sizing of the vessel and observation of lesion response to angioplasty. Postangioplasty angiography should then be done and unless an excellent result with 30% residual stenosis is seen, stenting should be performed with a stent size no larger than the smallest normal reference vessel segment proximal and distal to the lesion. The length of the stent should be kept to the minimum needed to cover the lesion or the angioplasty segment since longer stents are more difficult to deliver. Poststenting dilation is rarely needed unless a self-expanding stent is used; this last point is controversial and based on anecdotal experience with the Wingspan™ stent system (see below). If a large branch or perforator emanates from the lesion, then the increased risk of branch occlusion and consequent stroke should be discussed with the patient preprocedure. If this occurs, the authors have found, anecdotally, that intra-arterial infusion of glycoprotein (GP)IIb/IIIa antagonist may recanalize the occluded branch.
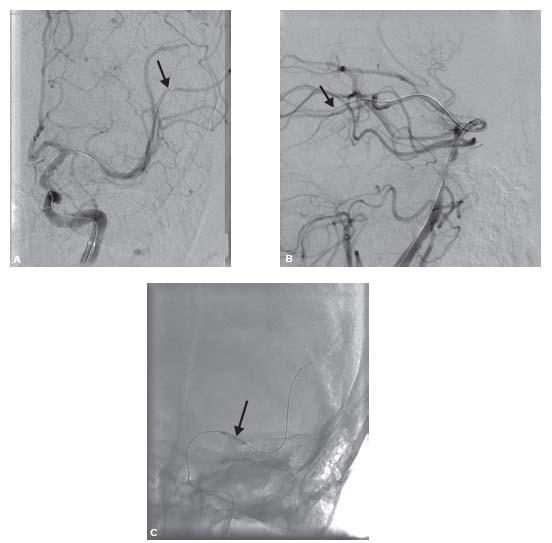
Figure 13B.1 • A: Middle cerebral artery (MCA) intervention with the wire tip (arrow) in M2 branch of the MCA. B: Basilar artery intervention with a wire (arrow) in the left posterior cerebral artery (PCA). C: Predilation of the MCA lesion in (A) with the Gateway™ balloon (Boston Scientific Corporation) (arrow) before MCA stenting.
Restoration of the normal vessel lumen diameter is not required to significantly improve flow through the stenosis because flow is proportional to the fourth power of the radius. As a result, the angiographic end point of a smooth, normal-caliber lumen, while desirable, must be balanced with the knowledge that the cerebral vessels are very fragile and persistent attempts to achieve such a goal may easily lead to tragic complications of arterial rupture or dissection and intracerebral hemorrhage (ICH).
PERIOPERATIVE MANAGEMENT
Because of the need for constant neurologic assessment of patients undergoing intracranial intervention, the author recommends that these procedures should not be performed under general anesthesia (10). If there is any clinical deterioration, angiography of the appropriate vessel should be performed immediately. Vasospasm, embolization, and dissection are the most likely etiologies of intraoperative deficits and should be treated appropriately. If angiography is normal but the patient has a clear neurologic deficit, an expanding ICH should be suspected and appropriate measures need to be taken. If there is frank extravasation of contrast on angiography, immediate blood pressure lowering, heparin reversal, and even temporary balloon occlusion should be considered. If there is no angiographic evidence of extravasation but a hematoma is suspected, intraoperative computerized tomography should be performed immediately. Under these circumstances, the authors have seen only a few patients survive despite all of the measures mentioned. If there are no new neurologic deficits, heparin may be discontinued at the end of the procedure but not reversed except in those who are at high risk for hyperperfusion syndrome or ICH. Routine use of GPIIb/IIIa antagonists is discouraged unless patients are inadequately premedicated with antiplatelet agents.
POSTOPERATIVE CARE
Close observation of neurologic status and monitoring of blood pressure are critical. If there is a risk of hyperperfusion syndrome and ICH, blood pressure should be kept in the low normal range for at least 14 days. Dual antiplatelet therapy needs to be continued for at least 30 days but may be continued for 6 to 12 months or until a follow-up angiogram confims that there is no restenosis. If drug-eluting stents (DES) were placed, prolonged therapy for 1 to 2 years may be necessary. In addition, all patients should have a 30-day follow-up with a transcranial Doppler ultrasound (TCD) and neurologic examination. At 6 months, another follow-up is needed, and unless the stented segment is easily evaluated by TCD, angiography should be done to assess stent patency. The authors have found it useful to know whether any early, severe neointimal proliferation occurs; in such cases, more frequent clinical assessments and continued dual antiplatelet therapy are warranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


