In 2006, approximately 350,000 patients were managed on dialysis programs in the United States alone, and this number is estimated to increase to 530,000 by 2020 (1,2). Almost 93% of these individuals are managed with hemodialysis (1,2). This has created an enormous cost and resource burden on the health care system. A large component of care of the dialysisdependent patient involves the creation and maintenance of appropriate access for adequate hemodialysis. In the past several decades, interventional practitioners have assumed an increasingly important role in the evaluation and treatment of access dysfunction. Percutaneous intervention is now generally accepted as first line treatment for the majority of access management.
Although this chapter focuses on the interventional practice of dialysis access management, the importance of a team approach to these patients cannot be overemphasized. Dialysis patients are complex and require coordination between multiple services including nephrologists, surgeons, interventionalists, and dialysis centers. Local expertise in various treatment modalities plays an important role in selection of the most appropriate management strategy. Multidisciplinary conferences and close communication between services that care for dialysis patients will lead to better patient outcomes (3).
LONG-TERM HEMODIALYSIS ACCESS
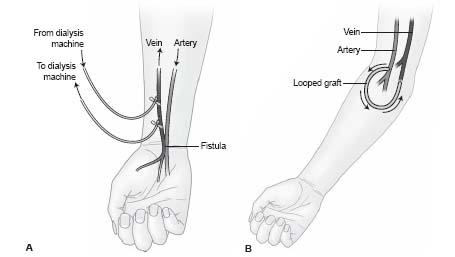
The two main types of long-term hemodialysis access are native fistulas and synthetic dialysis grafts (Fig. 28.1). These are surgically created connections between arteries and veins that create a long-term, high flow conduit to allow for hemodialysis. The National Kidney Foundation’s Kidney Disease Outcome Quality Initiative (NKF-KDOQI) guidelines have recommended that fistula be the primary access created in hemodialysis patients given their higher long-term patency, lower risks of infection, and lower cost of maintenance (4). Although fistula placement is increasing in frequency in the United States, dialysis grafts remain the most common longterm access.
Creation of dialysis access is performed first in the upper extremities, beginning in the non-dominant arm distally near the wrist and progressing more centrally to the upper arm to preserve the useable “venous capital” required for the creation of long-term access. When a single arm is exhausted of sites, the opposite arm is utilized in a similar fashion. When upper extremity sites for potential access have been exhausted, the lower extremities are utilized, although there is a greater risk of infection and lower long-term patency.
The most common fistulais the Brescia–Cimino fistula, which is an end-to-side anastomosis of the cephalic vein with the radial artery at the level of the wrist. A brachiocephalic fistula is the next most common fistula access, which is an end-to-side anastomosis of the cephalic vein to the brachial artery at the level of the elbow. Creation of these types of fistula requires mobilization of a short venous segment, and most side branches of the normal venous anatomy are left intact. This is in contrast with transposed fistula where a long segment of vein is exposed, the side branches are ligated, and the vein may be super ficialized in a staged procedure for ease of cannulation. Fistula need a period of time, typically 6 to 12 weeks, to dilate and mature before they can be used for hemodialysis. A significant number of fistula (20% to 50%) fail to mature and may need further interventional evaluation and management. Percutaneous interventions on the arterial anastomosis should not be performed for at least 4 weeks due to the risk of perforating the surgical anastomosis.
Synthetic grafts are most commonly created out of polytetrafl uoroethylene (PTFE) and may be either looped or straight in configuration. Looped grafts are preferred. Grafts tend to have lower patency rates, although they can be used within 2 weeks of creation for dialysis. The most common forearm graft is a loop graft from the brachial artery to the brachial vein. When upper extremity access has been exhausted, grafts in the thigh are most often constructed from the common femoral artery to the common femoral vein in a loop configuration. Grafts frequently develop stenosis at the graft-venous anastomosis. They tend to be technically easier to declot as they are a single conduit without side branches.
EVALUATION OF SHUNT DYSFUNCTION
The primary goal of monitoring programs is to identify possible stenoses that may be contributing to inadequate dialysis and predispose to access thrombosis (5). Early detection of access dysfunction can decrease patient morbidity associated with poor dialysis, prevent thrombosis, avoid the need for temporary access, and allow for new access planning in a more timely fashion.
Many parameters are monitored by a dialysis center and include static and dynamic pressures, flow rates, and recirculation rates (4,5). Additionally, clinical factors such as increased bleeding time or an abnormal bruit or thrill may raise suspicion of access dysfunction (4,5).
Access History
The first step in evaluation and treatment of any patient referred for percutaneous evaluation is to review the access history. This includes a combination of review of the medical record and a conversation with the patient. Review of the relevant operative report, previous venography, and prior percutaneous interventions will provide a great deal of important information. Particularly important is the age of the access, size of a graft, previous areas of stenosis, size and type of previous balloons used, and durability of the previous intervention. This information can also direct the physical exam and help guide the best site to access for diagnosis and therapy.
Figure 28.1 • Schematic representation of the two primary hemodialysis access types. A: Arteriovenous fistulabetween radial artery and cephalic vein (i.e., Brescia-Cimino fistula). B: Arteriovenous graft.
Physical Exam
All physicians performing interventions on dialysis access should be familiar with the basic physical exam of hemodialysis access. This will help predict the site of stenosis, direct the location of access for evaluation and therapy, and allow the operator to assess for changes after therapy. The anastomosis can be easily located by finding the surgical scar. Normally, a fistula has a soft, easily compressible pulse, a continuous thrill and low pitched bruit at the arterial anastomosis (6). An AV graft tends to be less compressible and has a thrill evident along a broader area (6,7). With a venous stenosis, the proximal pulse becomes firm with a water-hammer quality, often causing enlargement of a fistula and loss of the thrill (5,6). Central to a stenosis (i.e., on the venous side), there may be a caliber change in a fistula, a systolic predominant thrill and high-pitched bruit at the point of stenosis, and a diminished distal pulse (5,6).
Central obstruction can manifest as arm swelling or with dilated chest wall collaterals. Pseudoaneurysms are easily visualized and palpated. Skin breakdown over aneurysm sites is also important to note as it may signify impeding rupture or infection.
With intervention, it is important to know which way venous out flow is directed. This is obvious with AV fistula, but may not be obvious with a looped AV graft. Grafts are typically constructed with the arterial limb medially and the vzenous limb laterally, although anatomy may dictate that this is reversed in certain patients. Patients are often aware of which limb is arterial and asking the patient is helpful. Compression of the mid-graft will also define the direction of flow, as pulsation should only be subsequently felt in the arterial limb (6). With experience, physical exam can be accomplished within several minutes, often during the consent process.
Angiographic Evaluation
Angiographic evaluation of dialysis access is critical to diagnosis and treatment. The entire circuit from the arterial anastomosis through the right atrium should be evaluated. Most evaluations can be performed via the graft or fistula safely without the need for arterial puncture. Exceptions include evaluation of suspected steal syndrome, evaluation of the non maturing fistula, and evaluation of high-grade anastomotic stenoses that cannot be crossed from the venous side. In these cases, brachial arterial puncture may be very helpful.
Many clues can direct the operator to the most appropriate site for the initial puncture to avoid multiple sites of access. The first clue is the indication for the intervention. High venous pressure and increased bleeding time suggest a venous stenosis, while low flow or high recirculation rates can imply either arterial or venous problems. Physical exam and realtime evaluation of the access with sonography can also reliably detect stenotic or thrombosed segments. Additionally, review of the medical record can demonstrate areas of previous stenosis, which are likely to recur.
In the absence of other clues, access should be made in the fistula/graft near the arterial anastomosis directed to venous out flow, as the majority of stenoses occur in the venous circuit. Buttonhole access points (i.e., specific points that are constant and used to provide access for dialysis) should be avoided as percutaneous intervention at or near these sites may compromise future use. Pseudoaneurysms should also be avoided as access at these sites may promote expansion and eventual perforation. In the loop graft, access can be made near the graft apex directed to venous outflow to maintain a straight path for the treatment of lesions centrally.
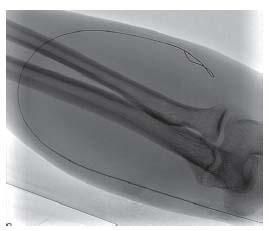
Figure 28.2 • Typical micropuncture of hemodialysis access visualized under fluoroscopy. The wire follows the expected course of the access and passes smoothly.
Micropuncture access with manual palpation or ultrasound guidance is recommended, as it is the least traumatic. For graft access, trapping the graft between two fingers during access is helpful. The fibrotic nature of grafts will yield a popping sensation as the needle penetrates the graft and pulsatile flow is observed. Access of fistula is similar, although it may be more difficult if the fistula is poorly developed or there is low flow. Use of upper arm tourniquets and real-time ultrasound is particularly useful. The passage of the guidewire should be observed with fluoros copy to ensure passage of the wire within the access (Fig. 28.2). Appropriately adjusting the patient under the image intensi-fier prior to access is advisable to prevent dislodging the needle during patient movement. Typically, the wire will pass easily without resistance and follow the expected course of the access. Any resistance should prompt evaluation of possible extravascular placement. This is easily accomplished with ultrasound (Fig. 28.3) and fluoroscopic evaluation.
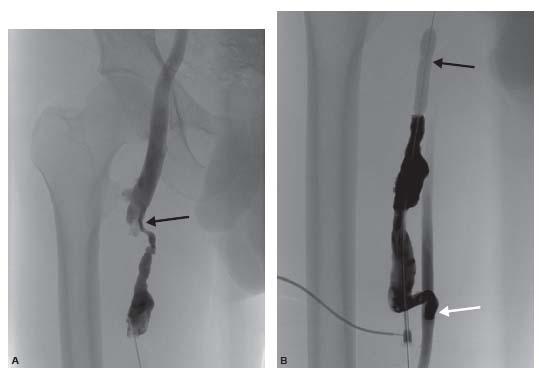
Diagnostic venography can then be performed through the 5-Fr. micropuncture transitional dilator used for access. Use of extension tubing and a one-way stopcock can decrease operator exposure during filming. The entire access circuit should be evaluated from the arterial anastamosis to the right atrium. The presence of collaterals is an important clue to a hemodynamically significant stenosis. Visualization of the arterial anastomosis can be accomplished by occlusion of the out flow during injection of contrast so that contrast Refluxes across the anastamosis. This can be performed by manual compression of the out flow or during balloon angioplasty of a venous stenosis by injection of the sheath side port (Fig. 28.4).
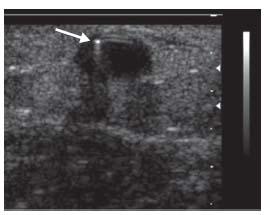
Figure 28.3 • Ultrasound guidance of hemodialysis access. Ultrasound easily demonstrates that access wire (white arrow) is appropriately within the vessel target.
The latter method decreases the radiation dose to the operator. Multiple obliquities are often needed to define the arterial anastomosis optimally. If the initial access is unacceptable for treatment, a new access is made in a similar fashion.
TREATMENT OF STENOSIS OF AV GRAFT OR MATURE AV FISTULA
Treatment of an angiographic stenosis in a graft or mature fistula is indicated when there is greater than 50% angiographic narrowing and some associated clinical or physiologic abnormality (low flow, elevated venous pressure, increased bleeding time, bruit, etc.) (4). The presence of venous collaterals is an important clue to hemodynamic significance and their resolution after successful angioplasty is further evidence that treatment is successful. Treatment is generally defined as successful if there is less than 30% residual stenosis.
After a diagnostic angiogram has been performed, a 6-Fr. sheath is placed, which will accommodate the majority of balloon sizes for the arm veins. The stenosis is crossed with an angled catheter and hydrophilic wire, utilizing roadmap technique if the stenosis is tight. The hydrophilic wire is replaced with a steel wire for the treatment as this simplifies exchanges and minimizes the risk of losing access. It is important to ensure that there is an adequate amount of wire purchase in the central vasculature to maintain access across the stenosis during device exchanges in case rupture occurs. The balloon diameter is typically mildly oversized to the adjacent normal vein by 10% to 15%. In the case of a graft, the balloon is sized to the graft diameter or oversized by 1 mm.
Balloon Selection
It is generally accepted that hemodialysis-related venous stenoses are resistant to balloon dilation. Many stenoses will respond to moderate-pressure (15 atm) angioplasty, although a subset of stenoses will require high pressures (30 atm). A routinely used high pressure balloon is the Conquest balloon (Bard Vascular, Tempe, AZ). Trerotola et al. found that almost 20% of stenoses in native fistula and 10% of stenoses in grafts required pressures greater than 20 atm (8). Given the greater cost of high-pressure balloons, the choice of the initial balloon is left to the operator, although in the face of recurrent stenosis, a high-pressure balloon may be a better initial choice. Typically, 4-cm-long balloons are used, although 2-cm-long balloons are helpful in areas of tortuosity, such as at the arterial anastomosis. Given the pressures required, an inflation device with a pressure gauge is mandatory. The balloon should be centered over the lesion and inflated under fluoroscopic guidance and the inflation pressure titrated to the patient’s response. Discomfort, pressure, and pain are common and dilation should be accompanied by conscious sedation. If the patient experiences significant pain, inflation should be stopped temporarily as this may indicate rupture. Gauging the patient’s pain response comes with experience, and frequent intermittent venography is recommended for dilation in the patient with significant pain. The stenosis will appear as a waist in the balloon and will slowly efface with increasing pressure. Watermelon seeding, where the balloon tends to push forward or backward on the wire, is common and should be guarded against. Inattention to this phenomenon may increase the risk of rupture. Once the balloon is at profile, it is typically safe to increase the pressure to the rated maximum burst pressure without an increase in the rupture rate or significantly changing the patient’s perceived pain. inflations of 60 to 120 seconds are typically performed, although several prolonged inflations of up to 5 minutes may be necessary to dilate resistant stenoses. During this inflation period, it is common to see the pressure on the inflation gauge drop as the elastic stenosis is being dilated. Maintaining inflation to the maximal burst pressure may be helpful to dilate resistant stenoses during these episodes of prolonged inflation. When the dilation is complete, the balloon is completely deflated by fluoroscopy and removed from the sheath. The balloon is left on the wire in case extravasation is detected and rapid balloon occlusion is required. Follow-up venography is performed through the sheath side port and assessment of success is judged by degree of residual stenosis and resolution of collateral veins. Treatment is generally defined as successful if there is less than 30% residual stenosis associated with a reduction of collateral filling (4). If there is a persistent narrowing that requires treatment, two to three prolonged additional inflations with the same balloon or changing to a higher-pressure balloon is recommended.
Figure 28.4 • Evaluation of the arterial anastomosis during balloon treatment of venous stenosis in a patient with a prior right thigh transposition fistula. A: A right thigh transposition fistulavenogram demonstrates a high grade venous anastomotic stricture (black arrow). B: During balloon inflation (black arrow), a Reflux venogram is performed through the sheath side port to demonstrate the arterial anastomosis (white arrow).
Elastic stenoses are defined as stenoses that persist after the waist has been completely effaced with balloon angioplasty. With appropriate oversizing (10% to 15%), use of high-pressure balloons (up to 30 atm), and prolonged inflation (2 to 5 minutes), resistant stenoses are rare. In these cases, cutting balloons or stents may be utilized.
Cutting balloons were first introduced in 1991. Vorwerk first described their use in the venous circulation in 1995 (9). Peripheral cutting balloons (Boston Scientific, San Diego, CA) have four small linear blades infolded into the balloon that create controlled incisions on the vascular wall (9). This differs from the irregular intimal injury caused by standard angioplasty. The working height of the blades when the balloon is fully expanded is only 0.007″. The controlled intimal injury created by cutting balloons allows for dilation of stenoses at lower atmospheric pressures and has been shown to reduce the inflammatory response in the vascular wall. This has been theorized to reduce intimal hyperplasia and potentially reduce the risk of restenosis.
The literature regarding cutting balloon angioplasty for dialysis access is sparse. Most studies are retrospective and many are confounded by the concurrent use of standard balloon angioplasty either before or after the use of cutting balloons. The only prospective study by Vesely and Siegel compared primary cutting balloon angioplasty (without concurrent standard percutaneous transluminal angioplasty (PTA)) to standard balloon angioplasty in hemodialysis grafts and found no significant differences in 6-month patency (10). The cutting balloon, therefore, does not seem to improve patency when all stenoses in grafts are considered. However, a study by Guiu et al. suggests that primary cutting balloon angioplasty may have improved patency in long segment stenoses in fistula (>2 cm), although the patient population in this study was small and the data have not been confirmed in larger prospective series (11).
Given their increased cost and a lack of compelling data, cutting balloons cannot be routinely recommended as first-line treatment for most stenoses. They may, however, be most effective for treating long segment stenoses in fistula and for treating elastic stenoses resistant to standard balloon angioplasty.
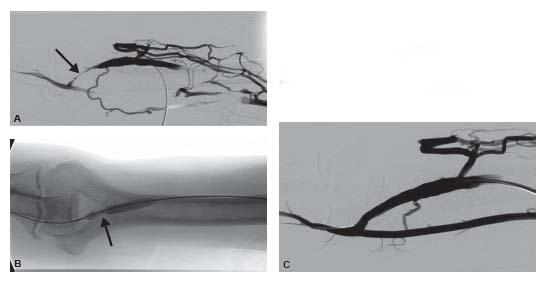
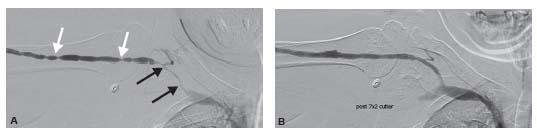
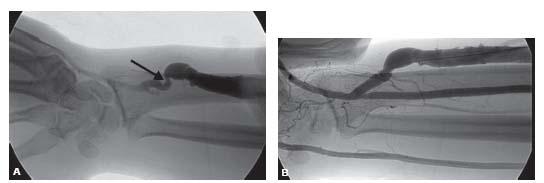
Figure 28.5 • Juxta-anastomotic stricture. A: A juxta-anastomotic stricture (black arrow) is present in an upper arm fistula. The venous collaterals are due to compression of the venous out flow and not from an underlying stenosis. B: The balloon waist (black arrow) is demonstrated during dilation. C: Post-angioplasty, there is complete resolution of the stenosis.
It is important to note that the technique of using cutting balloons differs from standard PTA in several ways. First, care must be taken not to grasp the balloon itself during insertion and withdrawal, as the operator may injure themselves on the blades. Cutting balloons are used over a 018″ wire system, and 5 to 8 mm diameter sizes are deliverable through a 7-Fr. sheath. Nominal inflation pressure is 6 atm, and the burst pressure is 10 atm. Given a theoretically higher risk of perforation, these balloons should not be oversized by more than 10%. Cutting balloons are inflated and deflated at a much slower rate than standard balloons, with the goal of allowing appropriate unfolding (during inflation) and refolding (during deflation) of the blades. When the balloon is brought to profile, it does not need to be brought to maximal pressure as the scoring of the vessel surface has already occurred. The balloon is maximally deflated and under negative pressure prior to sheath withdrawal, as the sheath may be cut if appropriate care is not taken. In general, pain with cutting balloon angioplasty is minimally reduced compared to standard PTA.
Specific Areas of Stenosis
Graft-Vein Anastamosis
AV grafts most commonly develop stenoses at the graft-vein anastomosis, although more central venous stenoses are also common. AV grafts are typically tubular and commonly measure 6 mm in diameter, although tapered grafts are used in many centers and can taper in diameter from 7 to 4 mm. Review of the operative note is helpful. Typically, it is best to size the balloon to the graft size or oversize it by 1 mm.
Juxta-Anastomotic Stenosis
Stenosis in the proximal several centimeters of a fistula is common and thought to be related to turbulent flow and some element of ischemia despite excellent surgical technique. These are known as juxta-anastomotic strictures (Figs. 28.5 and 28.6) and may be a common cause of failure of a fistulato mature.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree