VALVULAR HEART DISEASE
The role of the physical examination in the evaluation of patients with valvular heart disease is also considered in Chaps. 9 and 10; of electrocardiography (ECG) in Chap. 11; of echocardiography and other noninvasive imaging techniques in Chap. 12; and of cardiac catheterization and angiography in Chap. 13.
MITRAL STENOSIS
ETIOLOGY AND PATHOLOGY
Rheumatic fever is the leading cause of mitral stenosis (MS) (Table 20-1). Other less common etiologies of obstruction to left atrial outflow include congenital mitral valve stenosis, cor triatriatum, mitral annular calcification with extension onto the leaflets, systemic lupus erythematosus, rheumatoid arthritis, left atrial myxoma, and infective endocarditis with large vegetations. Pure or predominant MS occurs in approximately 40% of all patients with rheumatic heart disease and a history of rheumatic fever. In other patients with rheumatic heart disease, lesser degrees of MS may accompany mitral regurgitation (MR) and aortic valve disease. With reductions in the incidence of acute rheumatic fever, particularly in temperate climates and developed countries, the incidence of MS has declined considerably over the past few decades. However, it remains a major problem in developing nations, especially in tropical and semitropical climates.
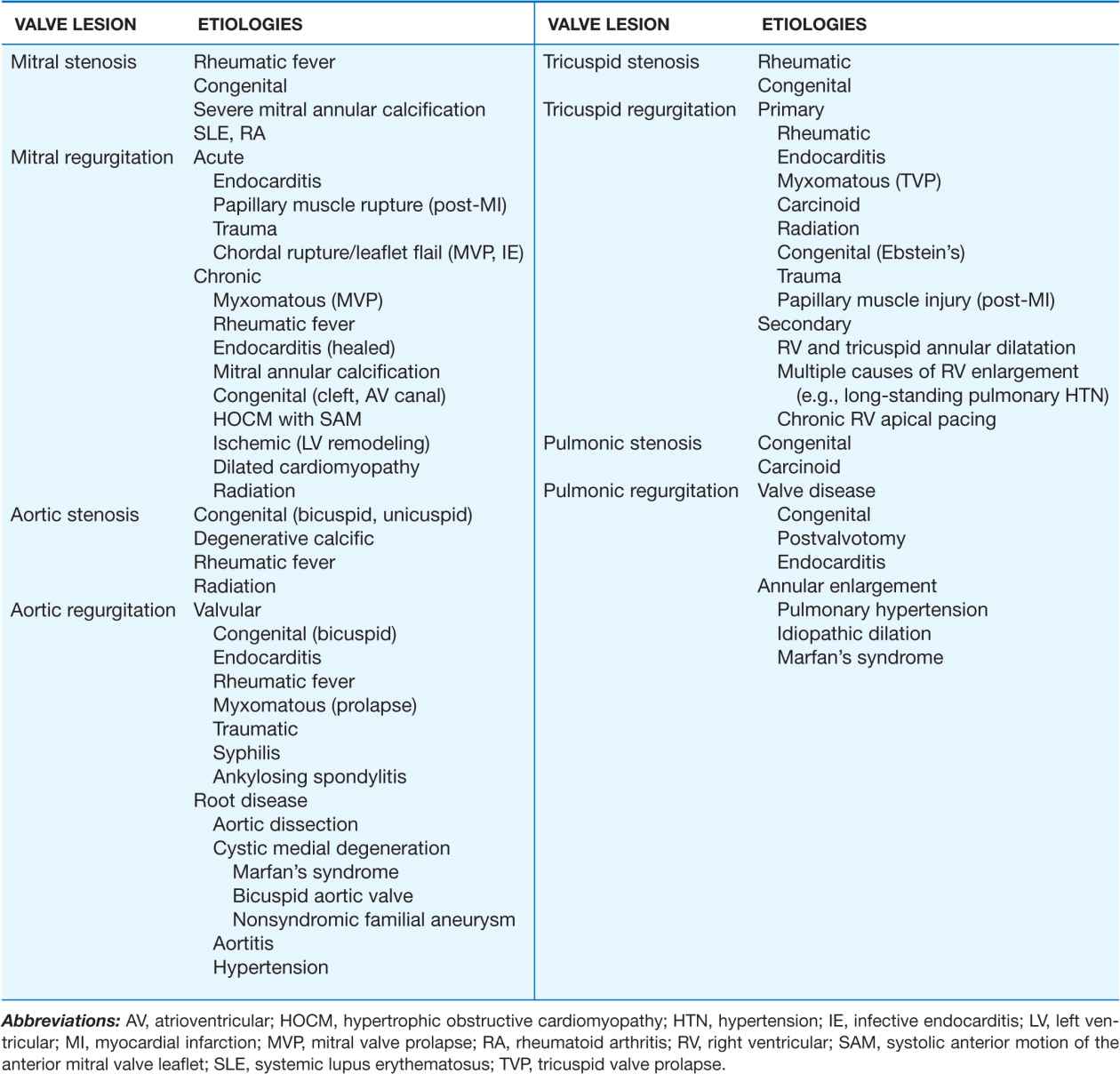
TABLE 20-1
MAJOR CAUSES OF VALVULAR HEART DISEASES
In rheumatic MS, the valve leaflets are diffusely thickened by fibrous tissue and/or calcific deposits. The mitral commissures fuse, the chordae tendineae fuse and shorten, the valvular cusps become rigid, and these changes, in turn, lead to narrowing at the apex of the funnel-shaped (“fish-mouth”) valve. Although the initial insult to the mitral valve is rheumatic, the later changes may be a nonspecific process resulting from trauma to the valve caused by altered flow patterns due to the initial deformity. Calcification of the stenotic mitral valve immobilizes the leaflets and narrows the orifice further. Thrombus formation and arterial embolization may arise from the calcific valve itself, but in patients with atrial fibrillation (AF), thrombi arise more frequently from the dilated left atrium (LA), particularly from within the left atrial appendage.
PATHOPHYSIOLOGY
In normal adults, the area of the mitral valve orifice is 4–6 cm2. In the presence of significant obstruction, i.e., when the orifice area is reduced to <~2 cm2, blood can flow from the LA to the left ventricle (LV) only if propelled by an abnormally elevated left atrioventricular pressure gradient, the hemodynamic hallmark of MS. When the mitral valve opening is reduced to <1 cm2, often referred to as “severe” MS, an LA pressure of ~25 mmHg is required to maintain a normal cardiac output (CO). The elevated pulmonary venous and pulmonary arterial (PA) wedge pressures reduce pulmonary compliance, contributing to exertional dyspnea. The first bouts of dyspnea are usually precipitated by clinical events that increase the rate of blood flow across the mitral orifice, resulting in further elevation of the LA pressure (see later in this chapter).
To assess the severity of obstruction hemodynamically, both the transvalvular pressure gradient and the flow rate must be measured (Chap. 13). The latter depends not only on the CO but on the heart rate, as well. An increase in heart rate shortens diastole proportionately more than systole and diminishes the time available for flow across the mitral valve. Therefore, at any given level of CO, tachycardia, including that associated with rapid AF, augments the transvalvular pressure gradient and elevates further the LA pressure. Similar considerations apply to the pathophysiology of tricuspid stenosis.
The LV diastolic pressure and ejection fraction (EF) are normal in isolated MS. In MS and sinus rhythm, the elevated LA and PA wedge pressures exhibit a prominent atrial contraction pattern (a wave) and a gradual pressure decline after the v wave and mitral valve opening (y descent). In severe MS and whenever pulmonary vascular resistance is significantly increased, the pulmonary arterial pressure (PAP) is elevated at rest and rises further during exercise, often causing secondary elevations of right ventricular (RV) end-diastolic pressure and volume.
Cardiac output
In patients with moderate MS (mitral valve orifice 1–1.5 cm2), the CO is normal or almost so at rest, but rises subnormally during exertion. In patients with severe MS (valve area <1 cm2), particularly those in whom pulmonary vascular resistance is markedly elevated, the CO is subnormal at rest and may fail to rise or may even decline during activity.
Pulmonary hypertension
The clinical and hemodynamic features of MS are influenced importantly by the level of the PAP. Pulmonary hypertension results from: (1) passive backward transmission of the elevated LA pressure; (2) pulmonary arteriolar constriction (the so-called “second stenosis”), which presumably is triggered by LA and pulmonary venous hypertension (reactive pulmonary hypertension); (3) interstitial edema in the walls of the small pulmonary vessels; and (4) at end stage, organic obliterative changes in the pulmonary vascular bed. Severe pulmonary hypertension results in RV enlargement, secondary tricuspid regurgitation (TR), and pulmonic regurgitation (PR), as well as right-sided heart failure.
SYMPTOMS
In temperate climates, the latent period between the initial attack of rheumatic carditis (in the increasingly rare circumstances in which a history of one can be elicited) and the development of symptoms due to MS is generally about two decades; most patients begin to experience disability in the fourth decade of life. Studies carried out before the development of mitral valvotomy revealed that once a patient with MS became seriously symptomatic, the disease progressed continuously to death within 2–5 years.
In patients whose mitral orifices are large enough to accommodate a normal blood flow with only mild elevations of LA pressure, marked elevations of this pressure leading to dyspnea and cough may be precipitated by sudden changes in the heart rate, volume status, or CO, as, for example, with severe exertion, excitement, fever, severe anemia, paroxysmal AF and other tachycardias, sexual intercourse, pregnancy, and thyrotoxicosis. As MS progresses, lesser degrees of stress precipitate dyspnea, the patient becomes limited in daily activities, and orthopnea and paroxysmal nocturnal dyspnea develop. The development of permanent AF often marks a turning point in the patient’s course and is generally associated with acceleration of the rate at which symptoms progress.
Hemoptysis results from rupture of pulmonary-bronchial venous connections secondary to pulmonary venous hypertension. It occurs most frequently in patients who have elevated LA pressures without markedly elevated pulmonary vascular resistances and is rarely fatal. Recurrent pulmonary emboli, sometimes with infarction, are an important cause of morbidity and mortality rates late in the course of MS. Pulmonary infections, i.e., bronchitis, bronchopneumonia, and lobar pneumonia, commonly complicate untreated MS, especially during the winter months.
Pulmonary changes
In addition to the aforementioned changes in the pulmonary vascular bed, fibrous thickening of the walls of the alveoli and pulmonary capillaries occurs commonly in MS. The vital capacity, total lung capacity, maximal breathing capacity, and oxygen uptake per unit of ventilation are reduced. Pulmonary compliance falls further as pulmonary capillary pressure rises during exercise.
Thrombi and emboli
Thrombi may form in the left atria, particularly within the enlarged atrial appendages of patients with MS. Systemic embolization, the incidence of which is 10–20%, occurs more frequently in patients with AF, in patients >65 years of age, and in those with a reduced CO. However, systemic embolization may be the presenting feature in otherwise asymptomatic patients with only mild MS.
PHYSICAL FINDINGS
Inspection and palpation
In patients with severe MS, there may be a malar flush with pinched and blue facies. In patients with sinus rhythm and severe pulmonary hypertension or associated tricuspid stenosis (TS), the jugular venous pulse reveals prominent a waves due to vigorous right atrial systole. The systemic arterial pressure is usually normal or slightly low. An RV tap along the left sternal border signifies an enlarged RV. A diastolic thrill may rarely be present at the cardiac apex, with the patient in the left lateral recumbent position.
Auscultation
The first heart sound (S1) is usually accentuated and slightly delayed. The pulmonic component of the second heart sound (P2) also is often accentuated, and the two components of the second heart sound (S2) are closely split. The opening snap (OS) of the mitral valve is most readily audible in expiration at, or just medial to, the cardiac apex. This sound generally follows the sound of aortic valve closure (A2) by 0.05–0.12 s. The time interval between A2 and OS varies inversely with the severity of the MS. The OS is followed by a low-pitched, rumbling, diastolic murmur, heard best at the apex with the patient in the left lateral recumbent position (see Fig. 9-5); it is accentuated by mild exercise (e.g., a few rapid sit-ups) carried out just before auscultation. In general, the duration of this murmur correlates with the severity of the stenosis in patients with preserved CO. In patients with sinus rhythm, the murmur often reappears or becomes louder during atrial systole (presystolic accentuation). Soft, grade I or II/VI systolic murmurs are commonly heard at the apex or along the left sternal border in patients with pure MS and do not necessarily signify the presence of MR. Hepatomegaly, ankle edema, ascites, and pleural effusion, particularly in the right pleural cavity, may occur in patients with MS and RV failure.
Associated lesions
With severe pulmonary hypertension, a pansystolic murmur produced by functional TR may be audible along the left sternal border. This murmur is usually louder during inspiration and diminishes during forced expiration (Carvallo’s sign). When the CO is markedly reduced in MS, the typical auscultatory findings, including the diastolic rumbling murmur, may not be detectable (silent MS), but they may reappear as compensation is restored. The Graham Steell murmur of PR, a high-pitched, diastolic, decrescendo blowing murmur along the left sternal border, results from dilation of the pulmonary valve ring and occurs in patients with mitral valve disease and severe pulmonary hypertension. This murmur may be indistinguishable from the more common murmur produced by aortic regurgitation (AR), although it may increase in intensity with inspiration and is accompanied by a loud and often palpable P2.
LABORATORY EXAMINATION
ECG
In MS and sinus rhythm, the P wave usually suggests LA enlargement (see Fig. 11-8). It may become tall and peaked in lead II and upright in lead V1 when severe pulmonary hypertension or TS complicates MS and right atrial (RA) enlargement occurs. The QRS complex is usually normal. However, with severe pulmonary hypertension, right-axis deviation and RV hypertrophy are often present.
Echocardiogram
(See also Chap. 12) Transthoracic echocardiography (TTE) with color flow and spectral Doppler imaging provides critical information, including measurements of mitral inflow velocity during early (E wave) and late (A wave in patients in sinus rhythm) diastolic filling, estimates of the transvalvular peak and mean gradients and of the mitral orifice area, the presence and severity of any associated MR, the extent of leaflet calcification and restriction, the degree of distortion of the subvalvular apparatus, and the anatomic suitability for percutaneous mitral balloon valvotomy (percutaneous mitral balloon valvuloplasty [PMBV]; see later). In addition, TTE provides an assessment of LV and RV function, chamber sizes, an estimation of the pulmonary artery pressure (PAP) based on the tricuspid regurgitant jet velocity, and an indication of the presence and severity of any associated valvular lesions. Transesophageal echocardiography (TEE) provides superior images and should be employed when TTE is inadequate for guiding management decisions. TEE is especially indicated to exclude the presence of left atrial thrombus prior to PMBV.
Chest x-ray
The earliest changes are straightening of the upper left border of the cardiac silhouette, prominence of the main pulmonary arteries, dilation of the upper lobe pulmonary veins, and posterior displacement of the esophagus by an enlarged LA. Kerley B lines are fine, dense, opaque, horizontal lines that are most prominent in the lower and midlung fields and that result from distention of interlobular septae and lymphatics with edema when the resting mean LA pressure exceeds approximately 20 mmHg.
DIFFERENTIAL DIAGNOSIS
Like MS, significant MR may also be associated with a prominent diastolic murmur at the apex due to increased antegrade transmitral flow, but in patients with isolated MR, this diastolic murmur commences slightly later than in patients with MS, and there is often clear-cut evidence of LV enlargement. An opening snap and increased P2 are absent, and S1 is soft or absent. An apical pansystolic murmur of at least grade III/VI intensity as well as an S3 suggest significant MR. Similarly, the apical mid-diastolic murmur associated with severe AR (Austin Flint murmur) may be mistaken for MS but can be differentiated from it because it is not intensified in presystole and becomes softer with administration of amyl nitrite. TS, which occurs rarely in the absence of MS, may mask many of the clinical features of MS or be clinically silent; when present, the diastolic murmur of TS increases with inspiration.
Atrial septal defect (Chap. 19) may be mistaken for MS; in both conditions there is often clinical, ECG, and chest x-ray evidence of RV enlargement and accentuation of pulmonary vascularity. However, the absence of LA enlargement and of Kerley B lines and the demonstration of fixed splitting of S2 with a grade 2 or 3 midsystolic murmur at the mid to upper left sternal border all favor atrial septal defect over MS. Atrial septal defects with large left-to-right shunts may result in functional TS because of the enhanced diastolic flow.
Left atrial myxoma (Chap. 23) may obstruct LA emptying, causing dyspnea, a diastolic murmur, and hemodynamic changes resembling those of MS. However, patients with an LA myxoma often have features suggestive of a systemic disease, such as weight loss, fever, anemia, systemic emboli, and elevated serum IgG and interleukin 6 (IL-6) concentrations. The auscultatory findings may change markedly with body position. The diagnosis can be established by the demonstration of a characteristic echo-producing mass in the LA with TTE.
CARDIAC CATHETERIZATION
Left and right heart catheterization is useful when there is a discrepancy between the clinical and TTE findings that cannot be resolved with either TEE or cardiac magnetic resonance (CMR) imaging. The growing experience with CMR for the assessment of patients with valvular heart disease may decrease the need for invasive catheterization. Catheterization is helpful in assessing associated lesions, such as aortic stenosis (AS) and AR. Catheterization and coronary angiography are not usually necessary to aid in decision making about surgery in patients younger than 65 years of age, with typical findings of severe mitral obstruction on physical examination and TTE. In men older than 40 years of age, women older than 45 years of age, and younger patients with coronary risk factors, especially those with positive noninvasive stress tests for myocardial ischemia, coronary angiography is advisable preoperatively to identify patients with critical coronary obstructions that should be bypassed at the time of operation. Computed tomographic coronary angiography (CTCA) (Chap. 12) is now often used to screen preoperatively for the presence of coronary artery disease (CAD) in patients with valvular heart disease and low pretest likelihood of CAD. Catheterization and left ventriculography are indicated in most patients who have undergone PMBV or previous mitral valve surgery, and who have redeveloped limiting symptoms, especially if questions regarding the severity of the valve lesion(s) remain after echocardiography.
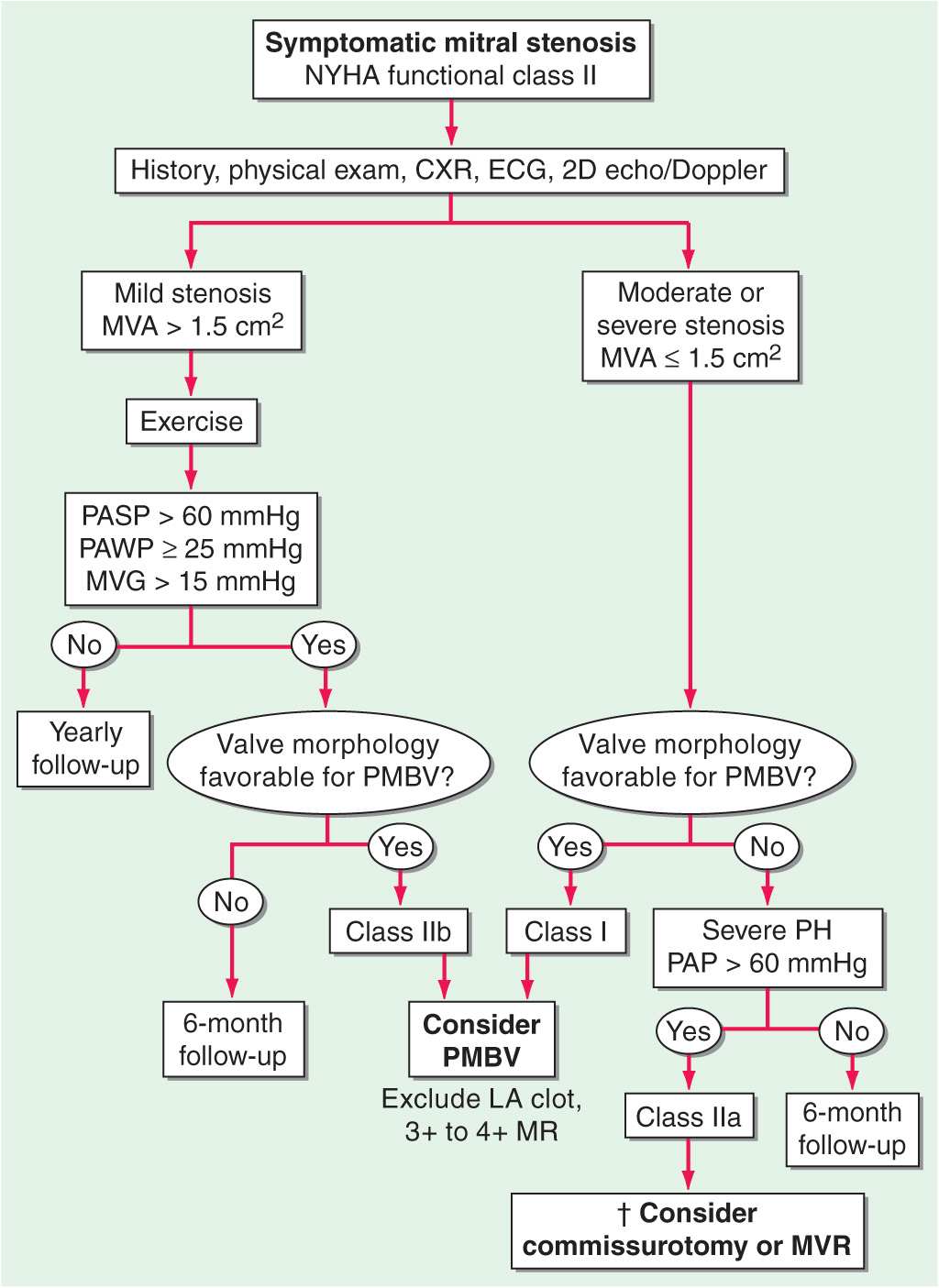
TREATMENT Mitral Stenosis
(See Fig. 20-1) Penicillin prophylaxis of group Aβ-hemolytic streptococcal infections (Chap. 26) for secondary prevention of rheumatic fever is important for at-risk patients with rheumatic MS (Table 20-2). Recommendations for infective endocarditis prophylaxis have recently changed. In symptomatic patients, some improvement usually occurs with restriction of sodium intake and small doses of oral diuretics. Beta blockers, nondihydropyridine calcium channel blockers (e.g., verapamil or diltiazem), and digitalis glycosides are useful in slowing the ventricular rate of patients with AF. Warfarin to an international normalized ratio (INR) of 2–3 should be administered indefinitely to patients with MS, who have AF or a history of thromboembolism. The routine use of warfarin in patients in sinus rhythm with LA enlargement (maximal dimension >5.5 cm) with or without spontaneous echo contrast is more controversial.
FIGURE 20-1
Management strategy for patients with mitral stenosis (MS) and mild symptoms. †There is controversy as to whether patients with severe MS (MVA <1 cm2) and severe pulmonary hypertension (PH) (PASP >60 mmHg) should undergo percutaneous mitral balloon valvotomy (PMBV) or mitral valve replacement (MVR) to prevent right ventricular failure. CXR, chest x-ray; ECG, electrocardiogram; echo, echocardiography; LA, left atrial; MR, mitral regurgitation; MVA, mitral valve area; MVG, mean mitral valve pressure gradient; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; PAWP, pulmonary artery wedge pressure; 2-D, two-dimensional. (From RO Bonow et al: J Am Coll Cardiol 48:e1, 2006; with permission.)
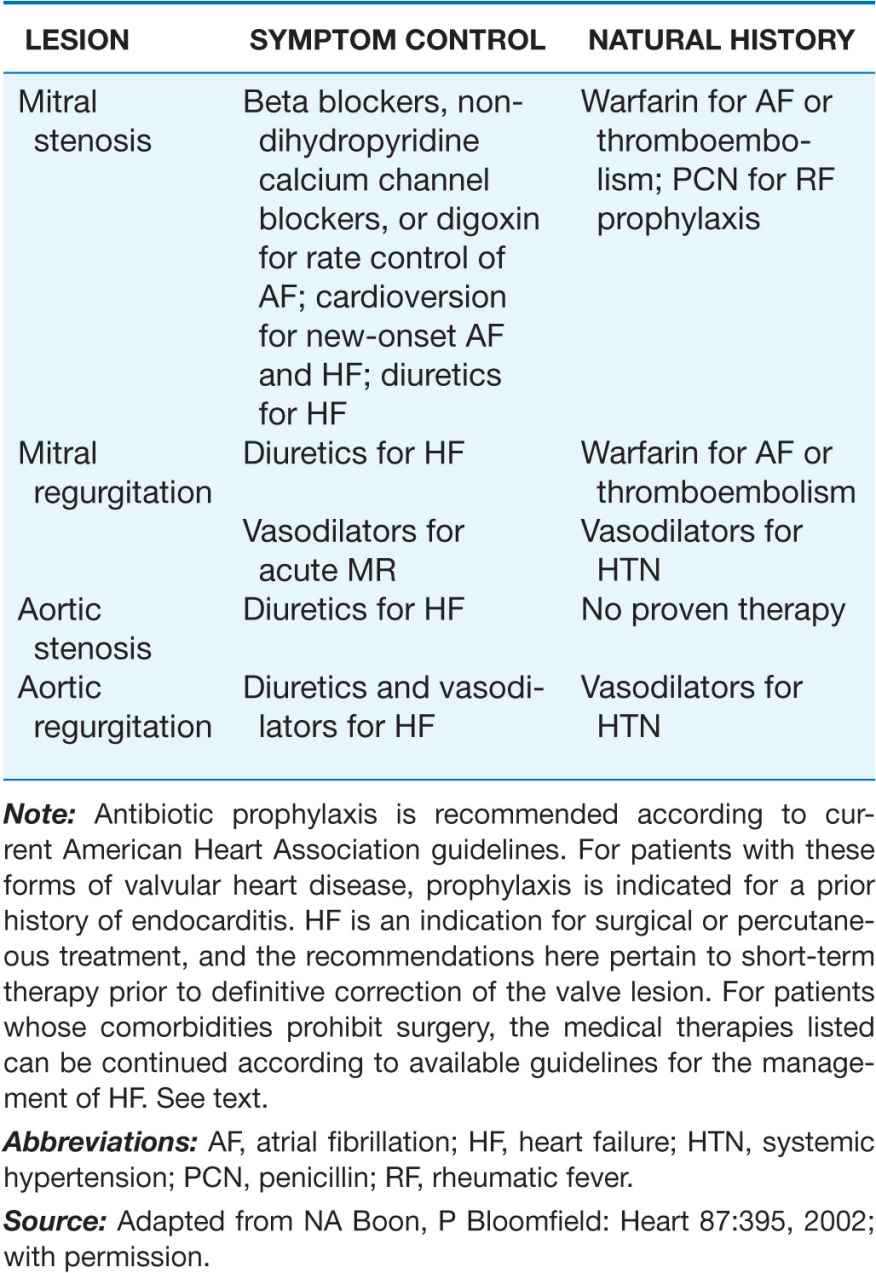
TABLE 20-2
MEDICAL THERAPY OF VALVULAR HEART DISEASE
If AF is of relatively recent onset in a patient whose MS is not severe enough to warrant PMBV or surgical commissurotomy, reversion to sinus rhythm pharmacologically or by means of electrical counter-shock is indicated. Usually, cardioversion should be undertaken after the patient has had at least 3 consecutive weeks of anticoagulant treatment to a therapeutic INR. If cardioversion is indicated more urgently, then intravenous heparin should be provided and TEE performed to exclude the presence of left atrial thrombus before the procedure. Conversion to sinus rhythm is rarely successful or sustained in patients with severe MS, particularly those in whom the LA is especially enlarged or in whom AF has been present for more than 1 year.
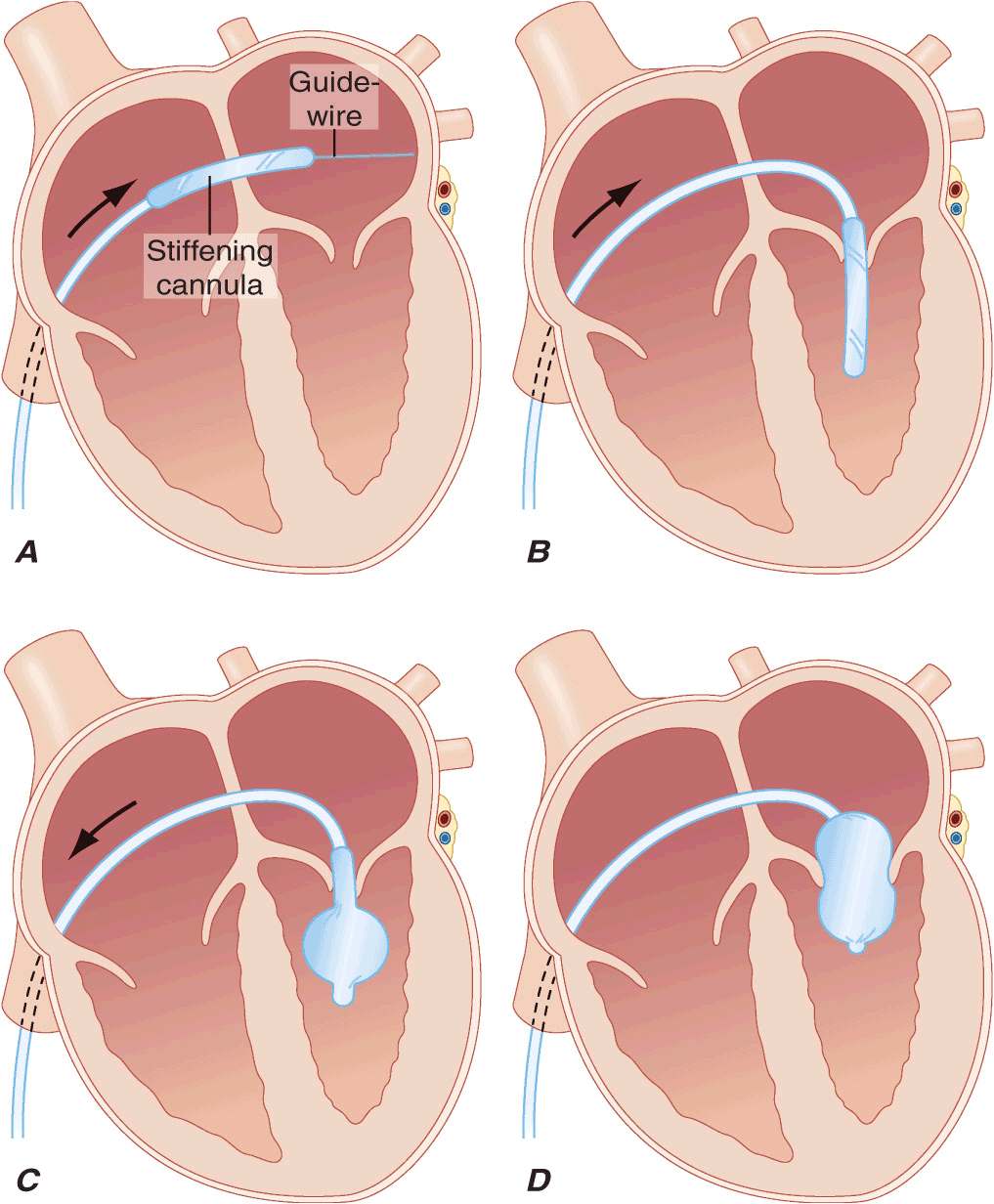
MITRAL VALVOTOMY Unless there is a contraindication, mitral valvotomy is indicated in symptomatic [New York Heart Association (NYHA) Functional Class II–IV] patients with isolated MS, whose effective orifice (valve area) is <~1 cm2/m2 body surface area, or <1.5 cm2 in normal-sized adults. Mitral valvotomy can be carried out by two techniques: PMBV and surgical valvotomy. In PMBV (Figs. 20-2 and 20-3), a catheter is directed into the LA after transseptal puncture, and a single balloon is directed across the valve and inflated in the valvular orifice. Ideal patients have relatively pliable leaflets with little or no commissural calcium. In addition, the subvalvular structures should not be significantly scarred or thickened, and there should be no left atrial thrombus. The short- and long-term results of this procedure in appropriate patients are similar to those of surgical valvotomy, but with less morbidity and a lower periprocedural mortality rate. Event-free survival in younger (<45 years) patients with pliable valves is excellent, with rates as high as 80–90% over 3–7 years. Therefore, PMBV has become the procedure of choice for such patients when it can be performed by a skilled operator in a high-volume center.
FIGURE 20-2
Inoue balloon technique for percutaneous mitral balloon valvotomy. A. After transseptal puncture, the deflated balloon catheter is advanced across the interatrial septum, then across the mitral valve and into the left ventricle. B–D. The balloon is inflated stepwise within the mitral orifice.
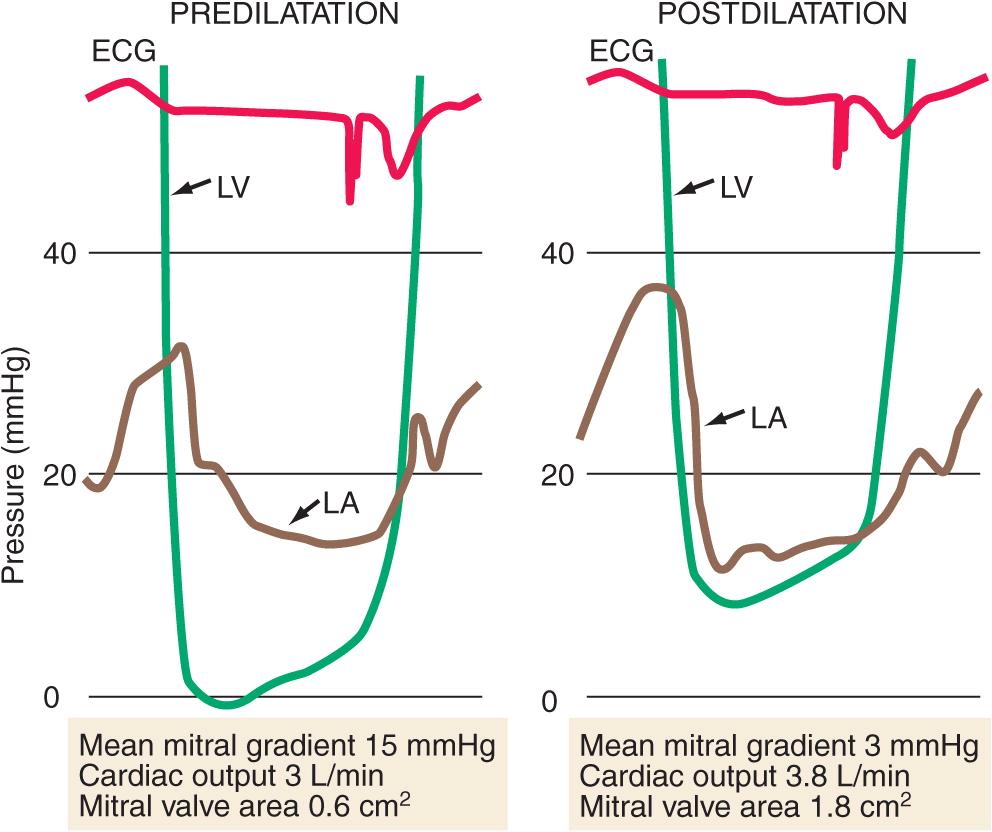
FIGURE 20-3
Simultaneous left atrial (LA) and left ventricular (LV) pressure before and after percutaneous mitral balloon valvuloplasty (PMBV) in a patient with severe mitral stenosis. (Courtesy of Raymond G. McKay, MD; with permission.)
Transthoracic echocardiography is helpful in identifying patients for the percutaneous procedure, and TEE is performed routinely to exclude left atrial thrombus at the time of the scheduled procedure. An “echo score” has been developed to help guide decision making. The score accounts for the degree of leaflet thickening, calcification, and mobility, and for the extent of subvalvular thickening. A lower score predicts a higher likelihood of successful PMBV.
In patients in whom PMBV is not possible or unsuccessful, or in many patients with restenosis, an “open” valvotomy using cardiopulmonary bypass is necessary. In addition to opening the valve commissures, it is important to loosen any subvalvular fusion of papillary muscles and chordae tendineae and to remove large deposits of calcium, thereby improving valvular function, as well as to remove atrial thrombi. The perioperative mortality rate is ~2%.
Successful valvotomy is defined by a 50% reduction in the mean mitral valve gradient and a doubling of the mitral valve area. Successful valvotomy, whether balloon or surgical, usually results in striking symptomatic and hemodynamic improvement and prolongs survival. However, there is no evidence that the procedure improves the prognosis of patients with slight or no functional impairment. Therefore, unless recurrent systemic embolization or severe pulmonary hypertension has occurred (PA systolic pressures >50 mmHg at rest or >60 mmHg with exercise), valvotomy is not recommended for patients who are entirely asymptomatic and/or who have mild stenosis (mitral valve area >1.5 cm2). When there is little symptomatic improvement after valvotomy, it is likely that the procedure was ineffective, that it induced MR, or that associated valvular or myocardial disease was present. About half of all patients undergoing surgical mitral valvotomy require reoperation by 10 years. In the pregnant patient with MS, valvotomy should be carried out if pulmonary congestion occurs despite intensive medical treatment. PMBV is the preferred strategy in this setting and is performed with TEE and no or minimal x-ray exposure.
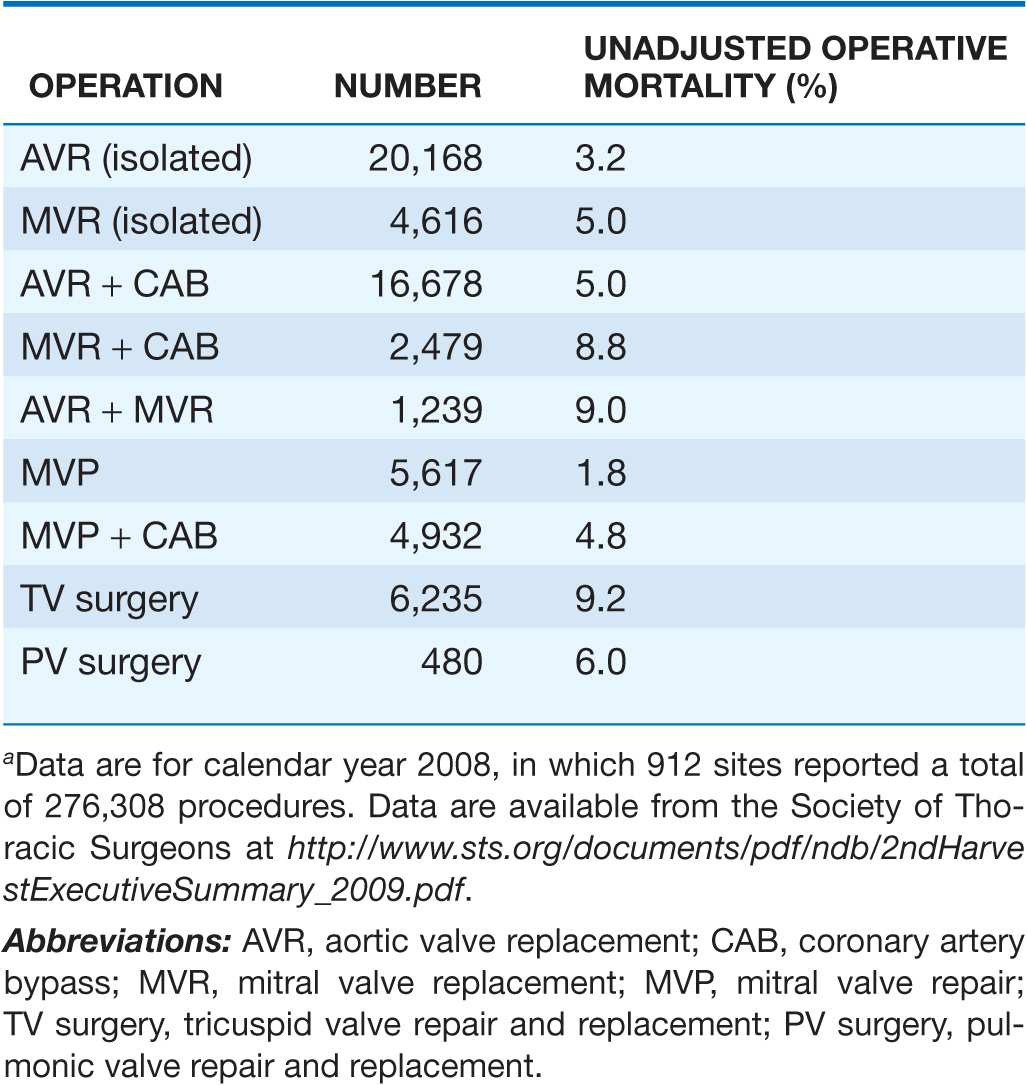
Mitral valve replacement (MVR) is necessary in patients with MS and significant associated MR, those in whom the valve has been severely distorted by previous transcatheter or operative manipulation, or those in whom the surgeon does not find it possible to improve valve function significantly with valvotomy. MVR is now routinely performed with preservation of the chordal attachments to optimize LV functional recovery. Perioperative mortality rates with MVR vary with age, LV function, the presence of CAD, and associated comorbidities. They average 5% overall but are lower in young patients and may be twice as high in patients >65 years of age with comorbidity rates (Table 20-3). Since there are also long-term complications of valve replacement, patients in whom preoperative evaluation suggests the possibility that MVR may be required should be operated on only if they have severe MS—i.e., an orifice area ≤1 cm2—and are in NYHA class III, i.e., symptomatic with ordinary activity despite optimal medical therapy. The overall 10-year survival of surgical survivors is ~70%. Long-term prognosis is worse in patients >65 years of age and those with marked disability and marked depression of the CO preoperatively. Pulmonary hypertension and RV dysfunction are additional risk factors for poor outcome.
MITRAL REGURGITATION
ETIOLOGY
MR may result from an abnormality or disease process that affects any one or more of the five functional components of the mitral valve apparatus (leaflets, annulus, chordae tendineae, papillary muscles, and subjacent myocardium) (Table 20-1). Acute MR can occur in the setting of acute myocardial infarction (MI) with papillary muscle rupture (Chap. 35), following blunt chest wall trauma, or during the course of infective endocarditis. With acute MI, the posteromedial papillary muscle is involved much more frequently than the anterolateral papillary muscle because of its singular blood supply. Transient, acute MR can occur during periods of active ischemia and bouts of angina pectoris. Rupture of chordae tendineae can result in “acute-on-chronic MR” in patients with myxomatous degeneration of the valve apparatus.
Chronic MR can result from rheumatic disease, mitral valve prolapse (MVP), extensive mitral annular calcification, congenital valve defects, hypertrophic obstructive cardiomyopathy (HOCM), and dilated cardiomyopathy (Chap. 21). The rheumatic process produces rigidity, deformity, and retraction of the valve cusps and commissural fusion, as well as shortening, contraction, and fusion of the chordae tendineae. The MR associated with both MVP and HOCM is usually dynamic in nature. MR in HOCM occurs as a consequence of anterior papillary muscle displacement and systolic anterior motion of the anterior mitral valve leaflet into the narrowed LV outflow tract. Annular calcification is especially prevalent among patients with advanced renal disease and is commonly observed in women >65 years of age with hypertension and diabetes. MR may occur as a congenital anomaly (Chap. 19), most commonly as a defect of the endocardial cushions (atrioventricular cushion defects). A cleft anterior mitral valve leaflet accompanies primum atrial septal defect. Chronic MR is frequently secondary to ischemia and may occur as a consequence of ventricular remodeling, papillary muscle displacement, and leaflet tethering, or with fibrosis of a papillary muscle, in patients with healed myocardial infarction(s) and ischemic cardiomyopathy. Similar mechanisms of annular dilation and ventricular remodeling contribute to the MR that occurs among patients with nonischemic forms of dilated cardiomyopathy once the left ventricular end-diastolic dimension reaches 6 cm.
Irrespective of cause, chronic severe MR is often progressive, since enlargement of the LA places tension on the posterior mitral leaflet, pulling it away from the mitral orifice and thereby aggravating the valvular dysfunction. Similarly, LV dilation increases the regurgitation, which, in turn, enlarges the LA and LV further, causing chordal rupture and resulting in a vicious circle; hence the aphorism, “mitral regurgitation begets mitral regurgitation.”
PATHOPHYSIOLOGY
The resistance to LV emptying (LV afterload) is reduced in patients with MR. As a consequence, the LV is decompressed into the LA during ejection, and with the reduction in LV size during systole, there is a rapid decline in LV tension. The initial compensation to MR is more complete LV emptying. However, LV volume increases progressively with time as the severity of the regurgitation increases and as LV contractile function deteriorates. This increase in LV volume is often accompanied by a reduced forward CO, although LV compliance is often increased and, thus, LV diastolic pressure does not increase until late in the course. The regurgitant volume varies directly with the LV systolic pressure and the size of the regurgitant orifice; as mentioned earlier, the latter, in turn, is influenced by the extent of LV and mitral annular dilation. Since ejection fraction (EF) rises in severe MR in the presence of normal LV function, even a modest reduction in this parameter (<60%) reflects significant dysfunction.
During early diastole, as the distended LA empties, there is a particularly rapid y descent in the absence of accompanying MS. A brief, early diastolic LA-LV pressure gradient (often generating a rapid filling sound [S3] and mid-diastolic murmur masquerading as MS) may occur in patients with pure MR as a result of the very rapid flow of blood across a normal-sized mitral orifice.
Semiquantitative estimates of left ventricular ejection fraction (LVEF), CO, PA systolic pressure, regurgitant volume, regurgitant fraction (RF), and the effective regurgitant orifice area can be obtained during a careful Doppler echocardiographic examination. These measurements can also be obtained with CMR. Left and right heart catheterization with contrast ventriculography is used less frequently. Severe, nonischemic MR is defined by a regurgitant volume ≥60 mL/beat, regurgitant fraction (RF) ≥50%, and effective regurgitant orifice area ≥40 cm2. Severe ischemic MR is usually associated with an effective regurgitant orifice area of >0.3 cm2.
LA compliance
In acute severe MR, the regurgitant volume is delivered into a normal-sized LA having normal or reduced compliance. As a result, LA pressures rise markedly for any increase in LA volume. The v wave in the LA pressure pulse is usually prominent, LA and pulmonary venous pressures are markedly elevated, and pulmonary edema is common. Because of the rapid rise in LA pressures during ventricular systole, the murmur of acute MR is early in timing and decrescendo in configuration ending well before S2, as a reflection of the progressive diminution in the LV-LA pressure gradient. LV systolic function in acute MR may be normal, hyperdynamic, or reduced, depending on the clinical context.
Patients with chronic severe MR, on the other hand, develop marked LA enlargement and increased LA compliance with little if any increase in LA and pulmonary venous pressures for any increase in LA volume. The LA v wave is relatively less prominent. The murmur of chronic MR is classically holosystolic in timing and plateau in configuration, as a reflection of the near-constant LV-LA pressure gradient. These patients usually complain of severe fatigue and exhaustion secondary to a low forward CO, while symptoms resulting from pulmonary congestion are less prominent initially; AF is almost invariably present once the LA dilates significantly.
SYMPTOMS
Patients with chronic mild-to-moderate isolated MR are usually asymptomatic. This form of LV volume overload is well tolerated. Fatigue, exertional dyspnea, and orthopnea are the most prominent complaints in patients with chronic severe MR. Palpitations are common and may signify the onset of AF. Right-sided heart failure, with painful hepatic congestion, ankle edema, distended neck veins, ascites, and secondary TR, occurs in patients with MR who have associated pulmonary vascular disease and marked pulmonary hypertension. Conversely, acute pulmonary edema is common in patients with acute severe MR.
PHYSICAL FINDINGS
In patients with chronic severe MR, the arterial pressure is usually normal, although the carotid arterial pulse may show a sharp upstroke owing to the reduced forward cardiac output. A systolic thrill is often palpable at the cardiac apex, the LV is hyperdynamic with a brisk systolic impulse and a palpable rapid-filling wave (S3), and the apex beat is often displaced laterally.
In patients with acute severe MR, the arterial pressure may be reduced with a narrow pulse pressure, the jugular venous pressure and wave forms may be normal or increased and exaggerated, the apical impulse is not displaced, and signs of pulmonary congestion are prominent.
Auscultation
S1 is generally absent, soft, or buried in the holosystolic murmur of chronic MR. In patients with severe MR, the aortic valve may close prematurely, resulting in wide but physiologic splitting of S2. A low-pitched S3 occurring 0.12–0.17 s after the aortic valve closure sound, i.e., at the completion of the rapid-filling phase of the LV, is believed to be caused by the sudden tensing of the papillary muscles, chordae tendineae, and valve leaflets. It may be followed by a short, rumbling, mid-diastolic murmur, even in the absence of structural MS. A fourth heart sound is often audible in patients with acute severe MR who are in sinus rhythm. A presystolic murmur is not ordinarily heard with isolated MR.
A systolic murmur of at least grade III/VI intensity is the most characteristic auscultatory finding in chronic severe MR. It is usually holosystolic (see Fig. 9-5A), but as previously noted it is decrescendo and ceases in mid- to late systole in patients with acute severe MR. The systolic murmur of chronic MR is usually most prominent at the apex and radiates to the axilla. However, in patients with ruptured chordae tendineae or primary involvement of the posterior mitral leaflet with prolapse or flail, the regurgitant jet is eccentric, directed anteriorly, and strikes the LA wall adjacent to the aortic root. In this situation, the systolic murmur is transmitted to the base of the heart and, therefore, may be confused with the murmur of AS. In patients with ruptured chordae tendineae, the systolic murmur may have a cooing or “seagull” quality, while a flail leaflet may produce a murmur with a musical quality. The systolic murmur of chronic MR not due to MVP is intensified by isometric exercise (hand grip) but is reduced during the strain phase of the Valsalva maneuver because of the associated decrease in LV preload.
LABORATORY EXAMINATION
ECG
In patients with sinus rhythm, there is evidence of LA enlargement, but RA enlargement also may be present when pulmonary hypertension is severe. Chronic severe MR is generally associated with AF. In many patients, there is no clear-cut ECG evidence of enlargement of either ventricle. In others, the signs of eccentric LV hypertrophy are present.
Echocardiogram
TTE is indicated to assess the mechanism of the MR and its hemodynamic severity. LV function can be assessed from LV end-diastolic and end-systolic volumes and EF. Observations can be made regarding leaflet structure and function, chordal integrity, LA and LV size, annular calcification, and regional and global LV systolic function. Doppler imaging should demonstrate the width or area of the color flow MR jet within the LA, the intensity of the continuous wave Doppler signal, the pulmonary venous flow contour, the early peak mitral inflow velocity, and the quantitative measures of regurgitant volume, RF, and effective regurgitant orifice area. In addition, the PA pressures can be estimated from the TR jet velocity. TTE is also indicated to follow the course of patients with chronic MR and to provide rapid assessment for any clinical change. The echocardiogram in patients with MVP is described in the next section. TEE provides greater detail than TTE (see Fig. 12-5).
Chest x-ray
The LA and LV are the dominant chambers in chronic MR. Late in the course of the disease, the LA may be massively enlarged and forms the right border of the cardiac silhouette. Pulmonary venous congestion, inter-stitial edema, and Kerley B lines are sometimes noted. Marked calcification of the mitral leaflets occurs commonly in patients with long-standing, combined rheumatic MR and MS. Calcification of the mitral annulus may be visualized, particularly on the lateral view of the chest. Patients with acute severe MR may have asymmetric pulmonary edema if the regurgitant jet is directed predominantly to the orifice of an upper lobe pulmonary vein.
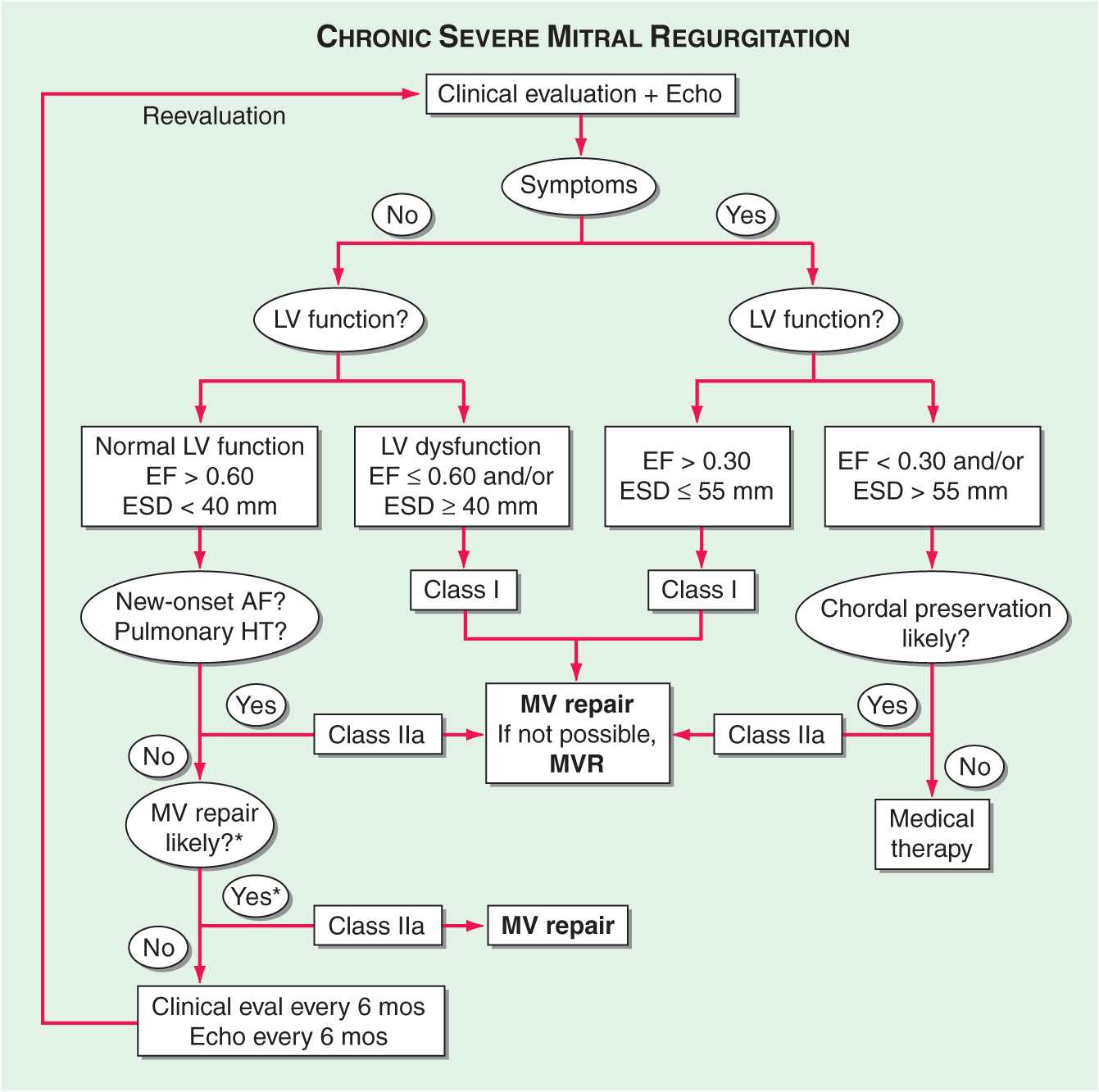
TREATMENT Mitral Regurgitation
MEDICAL TREATMENT (See Fig. 20-4 and Table 20-2) The management of chronic severe MR depends to some degree on its cause. Warfarin should be provided once AF intervenes with a target INR of 2–3. Cardioversion should be considered depending on the clinical context and left atrial size. In contrast to the acute setting, there are no large, long-term prospective studies to substantiate the use of vasodilators for the treatment of chronic, isolated severe MR with preserved LV systolic function in the absence of systemic hypertension. The severity of MR in the setting of an ischemic or nonischemic dilated cardiomyopathy may diminish with aggressive, evidence-based treatment of heart failure, including the use of diuretics, beta blockers, angiotensin- converting enzyme (ACE) inhibitors, digitalis, and biventricular pacing (cardiac resynchronization therapy [CRT]). Asymptomatic patients with severe MR in sinus rhythm with normal LV size and systolic function should avoid isometric forms of exercise.
Patients with acute severe MR require urgent stabilization and preparation for surgery. Diuretics, intravenous vasodilators (particularly sodium nitroprusside), and even intraaortic balloon counterpulsation may be needed for patients with post-MI papillary muscle rupture or other forms of acute severe MR.
SURGICAL TREATMENT In the selection of patients with chronic, nonischemic, severe MR for surgical treatment, the often slowly progressive nature of the condition must be balanced against the immediate and long-term risks associated with operation. These risks are significantly lower for primary valve repair than for valve replacement (Table 20-3). Repair usually consists of valve reconstruction using a variety of valvuloplasty techniques and insertion of an annuloplasty ring. Repair spares the patient the long-term adverse consequences of valve replacement, i.e., thromboembolic and hemorrhagic complications in the case of mechanical pros-theses and late valve failure necessitating repeat valve replacement in the case of bioprostheses. In addition, by preserving the integrity of the papillary muscles, subvalvular apparatus, and chordae tendineae, mitral repair and valvuloplasty maintain LV function to a relatively greater degree.
TABLE 20-3
MORTALITY RATES AFTER VALVE SURGERYa
Surgery for chronic, nonischemic, severe MR is indicated once symptoms occur, especially if valve repair is feasible (Fig. 20-4). Other indications for early consideration of mitral valve repair include recent-onset AF and pulmonary hypertension, defined as a PA pressure ≥50 mmHg at rest or ≥60 mmHg with exercise. Surgical treatment of chronic, nonischemic severe MR is indicated for asymptomatic patients when LV dysfunction is progressive, with LVEF falling below 60% and/or endsystolic dimension increasing beyond 40 mm. These aggressive recommendations for surgery are predicated on the outstanding results achieved with mitral valve repair, particularly when applied to patients with myxomatous disease, such as that associated with prolapse or flail leaflet. Indeed, primary valvuloplasty repair of patients younger than 75 years with normal LV systolic function and no CAD can now be performed by experienced surgeons with <1% perioperative mortality risk. Repair is feasible in up to 95% of patients with myxomatous disease. Long-term durability is excellent; the incidence of reoperative surgery for failed primary repair is ~1% per year for 10 years after surgery. For patients with AF, left or bi-atrial Maze surgery or radio-frequency isolation of the pulmonary veins is often performed to reduce the risk of recurrent, postoperative AF.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree