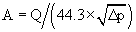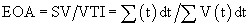CHAPTER 76 Valve Replacement Therapy
History, Options, and Valve Types
THE IDEAL VALVE
Many acceptable substitutes exist today for the replacement of diseased human heart valves. The ideal valvular prosthesis as described by Harken1 remains the Holy Grail of cardiac surgery. According to Harken, the ideal valve would be durable with a longevity approaching that of a native valve. Thrombogenicity would be nonexistent, and there would be no need for supplemental anticoagulation. In addition, the ideal replacement valve would have no inherent gradient, thus allowing unimpeded outflow. It also would be easily implanted and readily available. Finally, growth commensurate with that of the recipient would be possible.
HISTORY
The first human heart valve operation was a digital valvotomy of a stenotic aortic valve performed by Tuffier in 1914. Cutler, Souttar, Brock, Swan, and Harken refined valvotomies and commissurotomies in the ensuing decades. The need for a replacement valve arose out of the quest for an effective treatment of valvular insufficiency. In 1950, Hufnagel developed a ball valve, designed to be placed in the descending thoracic aorta. He was the first to implant a prosthetic valve in a human when he implanted his valve in the descending thoracic aorta of a patient with severe aortic insufficiency.2 In 1956, Gordon Murray implanted an aortic homograft in the descending thoracic aorta of a patient with severe aortic regurgitation.3 With the introduction of cardiopulmonary bypass, open valve replacement became a possibility. In 1960, Braunwald and Harken successfully performed mitral and aortic valve replacements with valves made of polyurethane.4 In 1961, Albert Starr, a surgeon, and Lowell Edwards, an engineer, developed a caged ball valve with which they achieved long-term survival.5 Caged ball valves worked well in many patients, but their high profile made implantation difficult in patients with small ventricular chambers and small aortic roots. High inherent gradients, along with a less favorable thromboembolic profile, made caged ball valves less desirable as new prostheses were introduced.
Several investigators designed disc valves, which functioned by having a disc pivot into an open or closed position as dictated by flow across the valve. The first of these tilting disc valves was the Wada hingeless valve introduced by the Japanese surgeon Jura Wada in 1966.6 The Lillehei-Kaster valve, a hingeless valve with a freely rotating pivoting disc retained by struts, was introduced in 1967 by C. Walton Lillehei and Robert L. Kaster. Viking Björk, working with Shiley Laboratories, developed a similar version of a hingeless pivoting disc valve. Although the hemodynamic profile was improved compared with the caged ball valves, these early pivoting disc valves were subject to occasional thrombosis. The 60-degree convexoconcave Björk-Shiley disc valve was prone to catastrophic structural failure secondary to fracture of its welded struts, allowing escape of the occluder disc.7 Seeking to improve on the problems of durability and thrombogenicity with these initial pivoting disc valves, Karl-Victor Hall, along with Woien and Kaster and the Medtronic Corporation, introduced the Medtronic-Hall valve in 1977.
The St. Jude Medical bileaflet mechanical prosthesis was also introduced in 1977.8 It has gone through several refinements and is currently the most commonly used mechanical valve prosthesis.
Independently, in 1969, Carpentier and Hancock developed the first porcine xenografts.9 Ionescu developed the first glutaraldehyde-preserved bovine pericardial valve in 1971.10
Limited long-term durability secondary to leaflet calcification and subsequent perforation plagued early bioprostheses. Significant progress has been made with the latest generation bioprostheses by state-of-the-art fixation and preservation methods to achieve extended durability. Stentless xenografts were introduced in 1986 as a way to counteract the inherent gradient found with stented bioprostheses.11
Ross reported the use of aortic homografts in 1962 and subsequently developed the Ross procedure, which uses a pulmonary autograft for aortic valve replacement.12
HEMODYNAMIC ASSESSMENT OF CARDIAC VALVES
The normal valve area is 3.0 to 4.0 cm2 for the aortic valve and 4.0 to 5.0 cm2 for the mitral valve. Stented bioprostheses and mechanical prostheses have inherent gradients because the actual valve is supported by a stent and housing material. The effective orifice area (EOA) refers to the true cross-sectional area of the prosthetic valve orifice through which blood must flow. The “gold standard” for measuring valve area is the Gorlin formula, which uses data derived from catheterization and applies hydraulic formulas for fixed orifice systems to heart valves.13 The formula is as follows:
The continuity equation has been shown to be accurate for bioprostheses.14 When it is applied to bileaflet valves, it has been shown to underestimate valve area because of localized high-velocity jets.15
Although valve manufacturers have often used the geometric cross-sectional area of prostheses to describe their size, the parameter that is clinically important is the functional orifice of the valve, the EOA. In general, EOA is proportional to valve size for a given type of prosthesis, and the EOA is generally always less than the cross-sectional area of a patient’s valve anulus. Accordingly, some degree of obstruction is always imparted by prostheses, which can be particularly important in the case of the aortic valve. Many surgeons and cardiologists believe that particularly for aortic valve replacement, the EOA of an implanted aortic prosthesis must be matched to the size of the patient to provide sufficient gradient relief. This is particularly the case during exercise. Although the concept of patient-prosthesis mismatch makes intuitive sense, its impact on patient morbidity and mortality in the short or long term remains unclear and is the subject of numerous studies. Some authors have suggested that patient-prosthesis mismatch can be avoided when the ratio of EOA to patient body surface area exceeds 0.85 cm2/m2. The manufacturer’s labeled valve size does not delineate the true internal or external diameter of a given valve and often does not represent the true EOA. Indeed, labeling of the valve sizes is arbitrary, and as such it cannot be used to compare values among different manufacturers and is not a true indicator of EOA.16
MORBIDITY AND MORTALITY GUIDELINES FOR VALVE OPERATIONS
Clinical studies are crucial for determining outcomes after cardiac valve operations, and precise definitions of outcomes are critical in comparing prostheses. To address this need, the councils of the Society of Thoracic Surgeons and the American Association for Thoracic Surgery formulated the Ad Hoc Liaison Committee for Standardizing Definitions of Prosthetic Heart Valve Morbidity. The initial report of this committee was issued in 1988,17 with an update following in 1996.18 The report strictly defines types of morbidity and mortality that can occur after valvular operations. An understanding of these definitions is crucial for interpreting studies dealing with valvular prostheses.
The guidelines distinguish two types of mortality: hospital mortality and 30-day mortality. Hospital mortality refers to death occurring at any time before discharge during a patient’s initial hospital stay. Thirty-day mortality, also referred to as operative mortality, is death that occurs at any time or place within 30 days of operation. There are several precise definitions of valve-related morbidity. Structural valve deterioration refers to “any change in function (a decrease of one New York Heart Association functional class or more) of an operated valve resulting from an intrinsic abnormality of the valve that causes stenosis or regurgitation.”18 It includes “changes intrinsic to the valve, such as wear, fracture, poppet escape, calcification, leaflet tear, stent creep, and suture line disruption of components … of an operated valve.”18 Thrombotic or infectious causes of valve dysfunction are not included.
Nonstructural dysfunction includes nonthrombotic and noninfectious causes of valvular stenosis or regurgitation that are not intrinsic to the valve itself. “Examples … include entrapment by pannus, tissue, or suture; paravalvular leak; inappropriate sizing or positioning; residual leak or obstruction from valve implantation or repair; and clinically important hemolytic anemia.”18 Morbid events are often reported as the composite linearized rate, or the number of events divided by the number of patient-years of follow-up (events/patient-years). The composite linearized rate for nonstructural dysfunction of the commonly available mechanical valves is 0.2 to 0.8 (events/patient-years) for the aortic position and 0.3 to 1.4 (events/patient-years) for the mitral position.19
Valve thrombosis is defined as thrombus in or about the valve that is not associated with infection and that interferes with valve function or obstructs blood flow through it. The composite linearized rate of thrombosis of mechanical valves is 0 to 0.2 (events/patient-years) for the aortic position and 0.4 to 0.8 (events/patient-years) for the mitral position.19
Embolism refers to any embolic event not associated with endocarditis that occurs after the immediate perioperative period and after the emergence from anesthesia. Embolic events are further delineated into neurologic events and peripheral embolic events. The composite linearized rate of thromboembolism ranges from 1.4 to 2.5 (events/patient-years) for the aortic position and 1.8 to 3.6 (events/patient-years) for the mitral position.19
A bleeding event refers to any clinically significant bleed requiring hospitalization or transfusion or causing death. A patient does not have to be taking an anticoagulant to sustain a bleeding event. Composite linearized rates vary from 0.8 to 2.5 (events/patient-years) for the aortic position and 1.2 to 2.2 (events/patient-years) for the mitral position.19
Endocarditis involving an operated valve is designated operated valvular endocarditis. “Morbidity associated with active infection, such as valve-thrombosis, thrombotic embolus, bleeding event, or paravalvular leak, is included under this category and is not included in other categories of morbidity.”18 Composite linearized rates for prosthetic valve endocarditis range from 0.4 to 0.7 (events/patient-years) for both the aortic and mitral positions.19
Consequences of morbid events are also defined by the guidelines. A reoperation is any operation on “a previously operated valve.”18 The composite linearized rate for reoperation ranges from 0.3 to 1.8 (events/patient-years) for the aortic position and 0.6 to 1.6 (events/patient-years) for the mitral position.19
Valve-related mortality is any death after a valve operation caused by a morbid event that is not related to progressive heart failure in patients with functioning valves. Unexplained deaths are just that and should be listed as such. Cardiac deaths include valve-related deaths, sudden deaths, and non–valve-related cardiac deaths. Total deaths refer to any and all deaths after a valve operation. “Permanent valve-related” impairment refers to any “permanent neurologic or functional deficit”18 caused by a morbid event.
PROSTHETIC HEART VALVES
Mechanical Prostheses
Caged Ball Prostheses
Starr-Edwards Silastic Ball Valve Prosthesis
The Starr-Edwards ball valve prosthesis (Edwards Lifesciences Corp., Irvine, CA) was introduced in 1966. The model currently available is constructed of a cage made from a single piece of titanium that encloses a Silastic ball. Although the valve is very durable and has a notable history, indications for its use today are limited because its hemodynamic and thromboembolic profiles do not match more modern designs (Fig. 76-1).19
Tilting Disc Prostheses
Medtronic-Hall Mechanical Heart Valve
The Medtronic-Hall tilting disc valve (Medtronic, Inc., Minneapolis, MN) was introduced in 1977 (Fig. 76-2). The valve housing is constructed from one piece of titanium alloy with no introduced welds or bends. The round central disc is made from tungsten-impregnated graphite with a pyrolytic carbon coating and has a central hole that allows the disc to be retained by a curved central guide strut that is part of the housing. It was designed to be an improvement on previous tilting disc valves in terms of durability, hemodynamic performance, and reduced thrombogenicity. Valve washing was improved by a relatively larger minor orifice and a disc that lifts out of the housing and rotates with opening.20 Loss of structural integrity has not been reported. The valve can be rotated after implantation and has a low inherent transvalvular gradient. It has a moderately high profile in the open position. Occluder impingement is possible because its position at the equator of the valve housing makes it susceptible to obstruction from retained valve elements, sutures cut too long, or pannus.
Aortic prostheses are available in sizes from 20 to 31 mm. The optimal orientation is with the larger orifice of the aortic prosthesis facing the greater curvature of the aorta. Mitral prostheses are available in sizes from 23 to 33 mm. Low incidence of valve-related morbidity and mortality has been demonstrated by several studies. Butchart and colleagues,20 from the University Hospital of Wales, reported their 20-year experience with 1766 Medtronic-Hall valve replacements. Akins,21 at the Massachusetts General Hospital, also reported his extensive experience with the Medtronic-Hall valve, with favorable results.
Omnicarbon Cardiac Valve Prosthesis
The Omnicarbon valve prosthesis (MedicalCV Inc., Inver Grove Heights, MN) is a low-profile tilting disc valve introduced in 1984. It is an evolution in design of the Lillehei-Kaster tilting disc valve and was introduced as an improvement over the Omniscience valve that was discontinued in 2000 secondary to concerns about high rates of thrombosis and reoperation. Whereas the Omniscience valve had a titanium housing, both the housing ring and tilting disc of the Omnicarbon valve are made of pyrolytic carbon. It can be rotated after implantation and has no fixed pivot grooves or recesses. The Omniscience design minimized diastolic regurgitation, which Edwards and colleagues,22 from the Wake Forest School of Medicine, postulated as the cause of the valve’s thromboembolic problems. The Omnicarbon valve is available in sizes from 19 to 33 mm in both aortic and mitral models. Studies in Japan and Europe23,24 have shown diminished complication rates compared with the Omniscience valve and morbidity rates that are comparable to those of other mechanical prostheses on the market today.
Monostrut Cardiac Valve Prosthesis
The Monostrut cardiac valve prosthesis (Alliance Medical Technologies Inc., Irvine, CA) is a third-generation tilting disc valve that is a descendant of the 60-degree convexoconcave Björk-Shiley tilting disc valve. Construction consists of a cobalt-based alloy orifice ring with an integral, nonwelded, monostrut retainer with a polytetrafluoroethylene sewing ring, a pyrolytic carbon disc occluder, and a 70-degree nominal opening angle.25 Aris and coworkers,26 in the 10-year report of the Spanish Monostrut Group, demonstrated 100% freedom from structural deterioration and a low rate of valve-related complications. Food and Drug Administration (FDA) approval occurred in 1997. The Monostrut is available in aortic sizes from 17 to 33 mm and mitral sizes from 27 to 33 mm. It is used in the Thoratec paracorporeal and intracorporeal ventricular assist device.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree