Introduction
The ideal replacement cardiac valve is yet to be developed. The ideal valve would be easy to implant, would be non-thrombogenic, would last the patient’ s lifetime, and in children it would grow with the child. Currently available mechanical and biologic heart valves are imperfect and heart valve repair should always be considered prior to replacement.
This chapter reviews the information on the surgical management of heart valve disease excluding transcatheter therapies. The heterogeneity of heart valve disease and the variability of outcome of complex procedures make the design and execution of randomized clinical trials difficult. The majority of the present evidence is derived from single-center reports. Our recommendations reflect the position of the American College of Cardiology and American Heart Association (ACC/AHA) 2006 Practice Guidelines.1
Since the publication of the Guidelines for Reporting Morbidity and Mortality After Cardiac Valvular Operations approved by the Society of Thoracic Surgeons and the American Association for Thoracic Surgery in 1988, and revised in 1996,2 surgical journals require that authors conform to those guidelines, which are presented in Box 58.1.
Natural history and indications for surgery
The ACC/AHA 2006 guidelines contain a comprehensive review of the natural history of heart valve disease and the indications for surgery.1 This information is also covered in Chapters 53–55, and 81.
Aortic valve repair
The morphologic characteristics and function of the aortic valve are interrelated to the aortic root and are best described as a single functional unit. The aortic root is the anatomic segment that connects the left ventricle with the ascending aorta and has four components: aortoventricular junction or aortic annulus, aortic cusps, aortic sinuses or sinuses of Valsalva, and sinotubular junction. Although the aortic cusps are the most important component of the aortic valve, aortic insufficiency (AI) can occur in patients with normal aortic cusps that have dilation of the sinotubular junction and/or aortic annulus.
Congenital aortic stenosis used to be managed by means of open surgical valvotomy but this procedure has been largely replaced by transcatheter valvotomy (see Chapter 56). Acquired aortic stenosis is not suitable for aortic valve repair.
Patients with AI are potential candidates for aortic valve repair and the aortic cusps are the most important determinant of reparability.3,4 If the cusps are thin and mobile and have smooth free margins, the feasibility of aortic valve repair is high. Calcified, fibrotic or excessively overstretched cusps preclude aortic valve repair. Aortic insufficiency caused by prolapse of a single cusp in patients with tricuspid aortic valves is uncommon but repairable. Prolapse of one cusp in patients with bicuspid aortic valve is common and is also suitable for repair.5 Aortic valve repair frequently is needed in combination with aortic valve-sparing operations.6 However, isolated repair of the aortic valve is not routinely carried out given the durability of contemporary mechanical and bioprosthetic valves (Class I, Level C).
BOX 58.1 Guidelines for reporting morbidity and mortality after cardiac valvular operations
Operative mortality
This is defined as death within the index hospitalization or within 30 days of the operation.
Structural valve deterioration
This is defined as any change in function (including a decrease in New York Heart Association functional class) of an operated valve resulting from an intrinsic abnormality of the valve that causes either stenosis or regurgitation. This definition, however, does not include deterioration because of infection or thrombosis of the valve as determined by reoperation, autopsy or clinical investigations. The term includes changes that are intrinsic to the valve such as wear, fracture, poppet escape, calcification, leaflet tear, stent creep, and suture line disruption of components of an operated valve.
Non-structural dysfunction
This is defined as any abnormality resulting in stenosis or regurgitation at the operated valve that is not intrinsic to the valve itself. Again, this term is exclusive of thrombosis and/or infection but includes entrapment by pannus, tissue or suture; paravalvular leak; inappropriate sizing or positioning; residual leak or obstruction; and clinically important hemolytic anemia.
Valve thrombosis
This includes any thrombosis, in the absence of infection, attached to or near an operated valve that occludes part of the blood flow path or that interferes with the function of the valve.
Embolism
This is any embolic event that occurs in the absence of infection after the immediate perioperative period (when anesthesia-induced unconsciousness is completely reversed). Importantly, a neurologic event includes any new, temporary or permanent focal or global neurologic deficit, but does not include psychomotor deficits elicited by specialized testing. Patients who do not awaken or who awaken after operation with a new stroke are excluded from tabulations of valve-related morbidity. A peripheral embolic event is also tabulated under this definition.
Bleeding event (formerly anticoagulant hemorrhage)
This is any episode of major internal or external bleeding that causes death, hospitalization or permanent injury or that requires blood transfusion. Notably, this definition applies also to patients who are not taking anticoagulants or antiplatelet agents.
Operated valvular endocarditis
This is defined as an infection involving an operated valve. It is based on the traditional criteria including an appropriate combination of positive blood cultures, clinical signs, and/or autopsy or operative findings. Importantly, morbidity associated with endocarditis, such as valve thrombosis, thrombotic embolus, bleeding event or paravalvular leak, is included under this category and is not included in other categories of morbidity.
Note added in proof: these guidelines have recently been updated. See Atkins CW et al. J Thorac Cardiovasc Surg 2008; 135(4):732–8.
Patients with aortic root aneurysm often have normal or minimally stretched aortic cusps and reconstruction of the aortic root with preservation of the native aortic cusps is feasible. Most patients with aortic root aneurysms have normally functioning aortic valve or mild AI. If the AI is severe, the cusps may be thinned and overstretched, or have stress fenestrations along the commissural areas. These valves are not suitable for repair. Patients with ascending aortic aneurysm and AI often have dilated sino-tubular junction and normal or minimally altered aortic cusps. The AI is central and caused by outward displacement of the commissures of the aortic valve.
Aortic valve-sparing operations are usually feasible in patients with aortic root aneurysms without AI or with AI and fairly normal aortic cusps as well as in patients with ascending aortic aneurysm and AI due to dilated sinotubular junction. These operations can be classified into three types7,8:
- replacement of the ascending aorta with downsizing of the sinotubular junction
- replacement of diseased aortic sinuses (one, two or all three sinuses – in the latter group the procedure is also referred to as a root remodeling or Yacoub procedure)9
- full root replacement with reimplantation of the aortic valve in a tube graft (David procedure).10
Aortic valve-sparing operations are technically demanding. They can be applied to aortic dissection that extends into the root,11 Marfan’ s syndrome patients with aortic root aneurysms,9,10 in patients with bicuspid aortic valves5,12 and in patients with aortic root aneurysms of unknown etiology. The competing options for dealing with aortic root and ascending aortic aneurysms are the composite valve graft (mechanical or bioprosthetic valve in a Dacron graft or a biologic valve conduit such as aortic valve homograft or xenograft)13,14 and aortic valve replacement and supra-coronary replacement of the ascending aorta.15,16
The long-term outcomes of aortic valve-sparing operations in patients with ascending aortic aneurysms are excellent.17 Reoperation rate at 10 years after replacement of the ascending aorta with reduction of the diameter of the sinotubular junction is only 3%, which is better than the results of valve replacement (Fig. 58.1). However, if the surgeon is not familiar with aortic cusp repair, presence of an eccentric AI jet at preoperative echocardiography should alert him or her to other concomitant aortic valve pathology and the need for more complex surgery.
Figure 58.1 Freedom from structural valve deterioration. If data from more than two reports were available, only data from the two largest reports were included. (Modified from Grunkemeier et al.110)
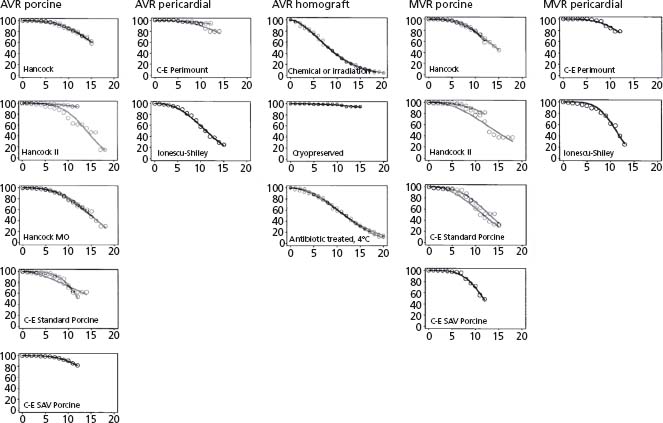
Aneurysm of the sinuses of Valsalva requires either a remodeling or a reimplantation procedure. Long-term results indicate a reoperation rate of about 10% at 10 years for progressive AI18 with the remodeling technique. In our own experience, return of moderate to severe AI occurred in 15% of patients at 10 years.6 This rate is suboptimal in young patients but may be acceptable in an older patient population.5 In patients with dilated aortic annuli, further strategies to stabilize the annulus should be employed.19 Otherwise, these patients, and patients with connective tissue disorders such as Marfan’ s syndrome, will have progressive annular dilation, which would cause AI again.6,20 The reimplantation technique allows stabilization of the annulus within the cylindrical graft and avoids moderate to severe AI in 94% of patients at 10 years.21,22 Some question the appropriateness of preserving the aortic valve in patients with Marfan’ s syndrome, but valve repair is safe and durable in this patient population.6,23 Composite valve graft replacement of the valve and aortic root remains the gold standard across most centers given the reproducibility of the procedure (Class I, Level C).
Mitral valve repair
The mitral valve, which includes the annulus, leaflets, chorda tendineae, papillary muscles, and left ventricular wall, may become dysfunctional for a variety of reasons.24 Rheumatic heart disease causes fibrosis of all components of the mitral valve with resulting mitral stenosis (MS) and/ or regurgitation (MR) (see Chapter 53). Myxomatous mitral valve disease typically causes MR because of elongated/ ruptured chordae and redundant and prolapsing leaflets. Ischemic functional MR is caused by infarction of the left ventricular wall that tethers the mitral leaflets. This causes an increase in the septolateral dimension of the annulus toward the posteromedial commissure or may result in leaflet prolapse due to papillary muscle rupture and/or fibrosis and elongation. Non-ischemic functional MR is caused by global left ventricular and mitral annular dilation.
Rheumatic mitral valve disease
Mitral balloon valvotomy, in the MS patient without MR and with low echocardiographic calcium scores, is the first line of intervention (see Chapter 57). Patients with moderate distortion of mitral valvular anatomy, but in whom the anterior leaflet and chordae tendineae still appear to be pliable, may be candidates for open mitral commissurotomy and repair. However, in patients in whom there is severe distortion of the valve apparatus preoperatively, mitral valve replacement is more appropriate. Repair of rheumatic MR has been relatively successful but may be attempted by experienced surgeons only if the mitral apparatus is not significantly damaged (Class IIa, Level C).
The failure of mitral valve repair in patients with rheumatic disease is related to progressive primary valve disease, leaftlet fibrosis and retraction.25 Valve repair in this population is not as durable as valve repair in patients with non-rheumatic disease. Freedom from reoperation is approximately 70–85% at 5–10 years of follow-up.26–29 A recent meta-analysis, however, demonstrated superior survival of patients with repair as compared to replacement.30 If a patient has an indication for anticoagulation, serious consideration should be given to mechanical mitral valve replacement (Class IIa, Level C). In this context, we emphasize the critical importance of preserving the chordae tendineae31–35 (Class I, Level C).
Myxomatous or degenerative mitral valve disease
The two major subcategories as initially defined by Carpentier are: myxomatous degeneration where typically the valve leaflets are redundant with elongated chordae, and fibroelastic deficiency which occurs in the older patient with minimal thickening of the leaflet.36 In the extreme form of myxomatous degeneration, the so-called “Barlow’s disease” of the mitral valve, there is also atrialization of the posterior mitral annulus that may have bearing on the technique of repair.37 Valve repair for these patients involves resection of the prolapsing segment,38 replacement and/or transposition of the chorda if necessary, and mitral valve annuloplasty.39,40 Mitral valve repair for degenerative mitral disease may be possible in greater than 90% of cases in experienced hands with excellent results and is recommended (Class I, Level C).
Several groups have reported long-term survival outcomes of mitral valve repair in comparison with mitral valve replacement.41–44 In broad terms, repair provides better survival and clinical outcomes than replacement, but more often than not, patients who undergo mitral valve replacement are sicker. The longest follow-up on mitral valve repair to date shows a freedom from reoperation of 92% at 20 years.45 At our center, freedom from reoperation ranged from 88% to 96% at 10 years depending on the prolapsing leaflet. Anterior leaflet prolapse or bileaflet prolapse portends worse outcome.46,47 Results of the edge-to-edge repair in a somewhat younger but similar patient population (with an incomplete follow-up) documented a freedom from reoperation of 96% at 10 years.48 The superior results obtained in the repair of the mitral valve in myxomatous disease,46 given the natural history of asymptomatic but severe MR,49 argues for early surgical intervention (Class IIa, Level C). Caution and judgement, however, must be exercised when the mitral valve is affected by rheumatic disease, if MR is caused by complex valvular abnormalities that include anterior leaflet prolapse, or if the surgical team has inadequate experience with valve repair.
Endocarditis
Mitral valve replacement had been the standard surgical therapy for patients with mitral valve endocarditis but repair is feasible depending on the degree of tissue destruction.50–52 A recent pooled analysis of the reported series demonstrated low rates of early and late repeat mitral valve surgery after mitral valve repair for endocarditis.53 The principles of mitral valve repair in endocarditis are to remove all infected tissue and to avoid implantation of foreign material. Autologous pericardium, which may be fixed with glutaraldehyde, or bovine pericardium can be used in place of prosthetic annuloplasty rings.
Ischemic mitral regurtitation (IMR)
The role of mitral valve replacement versus repair in chronic IMR is controversial.54 Mitral replacement is typically reserved for patients with higher preoperative risk factors and co-morbidity at experienced centers.55–57 Although the results of mitral valve repair appear to be superior to mitral valve replacements in early follow-up, these differences disappear and possibly reverse in longer follow-up.55–57 This suggests that mitral valve replacement may be the procedure of choice at centers that have limited experience with mitral valve repair (Class IIa, Level C).
The most common technique of mitral valve repair for IMR is undersized annuloplasty. Considering the asymmetric alteration in the mitral valve annulus, some advocate the use of a rigid and geometric ring that reduces the septal-lateral annular dimension in particular in the posteromedial segment.58 Clinical outcomes do not support the use of a complete over a partial ring59 but given that the intertrigonal region dilates over time, it is reasonable to suggest that complete rings are likely to provide better long-term durability.60,61 Most support the use of a rigid ring,62 although concerns that rigid ring annuloplasty may adversely affect LV function remain.63,64 The long-term result of mitral repair for IMR is poor with return of sig-nificant MR in nearly 30% of patients.59 Perhaps an undersized annuloplasty does not fully address the multifaceted alterations in the mitral apparatus of a patient with IMR.24 We have clinically introduced Levine’ s concept of severing secondary chordae tendineae.65,66 Our initial results are encouraging.65
Non-ischemic mitral regurgitation
The principal repair techniques are undersized annulo-plasty and the edge-to-edge repair. Correction of severe MR in end-stage heart failure is safe.67,68 In spite of symptomatic benefit, no survival benefit is currently apparent.69,70 Importantly, the Acorn clinical trial confirmed the impact of MR repair alone on patient symptoms and also documented progressive decline in left ventricular volume in a randomized trial setting71 (Class IIa, Level B). The results of the edge-to-edge technique in patients with LV dysfunction have been somewhat mixed.72,73
Tricuspid valve repair
Commonly, tricuspid regurgitation (TR) occurs as a result of left heart pathology. In these instances, the tricuspid apparatus is normal, but dilation of the tricuspid annulus results in significant regurgitation. Organic tricuspid valve disease is less common. Functional TR may be repaired by suture annuloplasty (De Vega procedure) or by ring annuloplasty. Ring annuloplasty provides superior long-term outcomes74–76 (Class IIa, Level C). Plication of the posterior leaflet (bicuspidalization) is now rarely performed.77
Types of heart valves
The two main categories of valves are mechanical and tissue valves. Within the former category, three principal valve types are available: caged-ball valves, single tilting disc valves, and bileaflet valves. Tissue valves may also be divided in to three major subtypes: xenografts, homografts, and pulmonary autograft. Xenograft valves are either porcine aortic valves or constructed pericardial valves. Xenograft valves can also be subdivided into stented and stentless.
Randomized clinical trials: mechanical versus biologic
Two randomized clinical trials have compared the outcome of aortic and mitral valve replacement. The Edinburgh Heart Valve Trial78–80 enrolled patients between 1975 and 1979 and the Department of Veterans Affairs Trial81–83 enrolled patients between 1977 and 1982. Both of these trials compared the Bjork–Shiley tilting disk valve to the first-generation Hancock or Carpentier–Edwards porcine valve. Although these valves are no longer used in North America, certain principles of valve behavior may be distilled from these two trials.
Edinburgh Heart Valve Trial
In this study, a total of 541 men and women undergoing either aortic or mitral valve replacement were included. The major finding of this trial was the lack of survival benefit for a particular valve type at 20 years. Porcine valves had a substantially higher structural valve deterioration and reoperation rate. Bioprosthesis had structural valve deterioration 12–14 years after implantation in the aortic position and 8–10 years after implantation in the mitral position. The bleeding rates were substantially higher with the mechanical valves, implanted in the aortic but not in the mitral position.
Department of Veterans Affairs Trial
This multicenter trial enrolled a total of 575 men only. It showed that patients randomized to receive a mechanical valve had improved survival after aortic valve replacement (AVR) but not after mitral valve replacement (MVR). Importantly, differences in mortality started to diverge after 10 years. As with the Edinburgh trial, porcine valves had substantially higher primary valve failure. The outcome in the two groups at 15 years is summarized in Table 58.1.
Table 58.1 Probability of an outcome event at 15 years after valve replacement
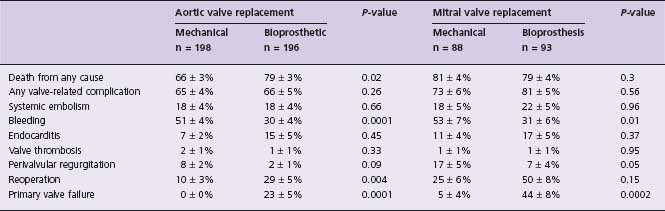
n, number of patients randomized; P, significance of difference between mechanical and bioprosthetic valve groups.
From Hammermeister et al.81
Differences between the trials
In the Edinburgh trial, the linearized rates of major hemorrhage requiring hospital admission and/or blood transfusions were 1.5% per year in the porcine group and 2% per year in the mechanical valve group. In the VA trial the linearized bleeding rates were 2.5% per year in the porcine group and 4.3% in the mechanical group. Higher intensity of anticoagulation in the USA likely accounts for this difference.84 In the contemporary data from the Stroke Prevention in Atrial Fibrillation III85 trial with international normalized ratio (INR) of 2.0–3.0, the incidence of bleeding was 1.5% per year. The linearized rates of thromboembolism were not different between the two types of valve.
New developments in valve prostheses
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


