Pulmonary hypertension (PH) is usually perceived as a complication of established heart failure (HF) rather than as a predictor of HF or a marker of subclinical HF. PH may develop because of cardiac alterations that result in increased filling pressures after acute myocardial infarction (AMI). We hypothesized that PH might be a useful marker to predict the risk of HF after AMI. We studied 1,054 patients with AMI. Pulmonary artery systolic pressure (PASP) was estimated using echocardiography at the index admission and PH was defined as a PASP >35 mm Hg. The primary end point was readmission for HF at 1 year. PH was present in 471 patients (44.6%) and was strongly associated with age, decreased ejection fraction, advanced diastolic dysfunction, and moderate/severe mitral regurgitation (p <0.0001 for all comparisons). Area under the receiver operating characteristic curve was significantly higher for estimated PASP (0.74 ± 0.02) compared to other echocardiographic parameters (p = 0.02 to 0.0003). After adjustments for clinical and echocardiographic variables in a Cox model, PH was associated with a hazard ratio of 3.10 for HF (95% confidence interval 1.31 to 2.57, p <0.0001). After adding estimated PASP to a model containing clinical and echocardiographic risk factors, net reclassification improvement was 0.21 (95% confidence interval 0.11 to 0.31, p <0.0001). In conclusion, PASP integrates the severity of multiple hemodynamic determinants of increased left atrial pressures that lead to an increase in pulmonary venous pressure. In AMI, PH at the index admission is a useful marker in unmasking latent subclinical HF and predicting the development of overt HF.
We hypothesized that pulmonary hypertension (PH), a marker of increased filling pressures, might be a useful marker to predict the risk of developing heart failure (HF) after acute myocardial infarction (AMI). To this end we performed a post hoc analysis using data from a prospective observational study designed to determine predictors of postinfarction HF. The database provided an opportunity to study the associations between estimated pulmonary artery systolic pressure (PASP) and HF in the setting of recent AMI, accounting for other important clinical and echocardiographic variables including left ventricular systolic function and severity of mitral regurgitation (MR). We present data that support the hypothesis that increased PASP as measured during the index hospitalization is a powerful predictor of incident HF after hospital discharge.
Methods
The study cohort consisted of patients enrolled in a prospective longitudinal observational study designed to determine predictors of postinfarction HF, with data collection starting in 2001. The study was approved by the investigational review committee on human research.
All patients presenting to the intensive coronary care unit with AMI were eligible for entry into the study if they had a diagnosis of AMI according to American College of Cardiology criteria. For the present analysis the exclusion criteria included (1) patients with evidence of previous HF and (2) patients in whom tricuspid regurgitation jets were not analyzable.
Echocardiography was performed during patients’ hospital stay after a median of 2 days from admission (interquartile range 1 to 3). Analysis of estimated PASP, left ventricular function, and presence and degree of MR was carried out by 1 of 5 experienced noninvasive cardiologists (Y.A., D.M., J.L., D.A., and S.C.) without knowledge of the patient outcome.
Estimated PASP was calculated as the sum of the peak systolic pressure gradient across the tricuspid valve (approximated by the modified Bernoulli equation) and right atrial pressure. Right atrial pressure was estimated according to the size and respiratory variation of the inferior vena cava diameter in the subcostal view. PH was defined as an estimated PASP >35 mm Hg.
Severity of MR was graded by color Doppler flow mapping, integrating jet expansion within the left atrium (jet area/atrial area), and jet eccentricity as previously described. Left ventricular ejection fraction was visually estimated and classified as normal (≥55%), mildly decreased (45% to 54%), moderately decreased (30% to 44%), or severely decreased (<30%). Left atrial dimensions were obtained using M-mode echocardiography guided by 2-dimensional imaging.
The primary end point of the study was rehospitalization for development of HF at 1 year. Hospitalization for HF or all-cause mortality was a secondary end point. HF was defined as readmission to the hospital for management of HF (defined by the presence of new symptoms of paroxysmal nocturnal dyspnea, orthopnea, or edema with ≥1 concurrent sign including ventricular gallop rhythm, jugular venous distention, bilateral post-tussive rales in at least the lower third of the lung fields, increased venous pressure, or pulmonary venous congestion on x-ray with interstitial or alveolar edema).
After hospital discharge, clinical end-point information was acquired by reviewing the national death registry and by reviewing hospital records for major clinical events if a patient had been rehospitalized. To confirm the diagnosis of HF, all hospital records were abstracted. Data were collected on the course and care of a patient during the hospital stay including admission notes, consultation notes, discharge summaries, and pertinent laboratory data.
Data are expressed as mean ± SD. Baseline characteristics and echocardiographic parameters of study groups were compared using unpaired t test for continuous variables and chi-square statistic for categorical variables.
Univariable and multivariable logistic regression analyses were performed to determine the relation between candidate variables and increased PASP (>35 mm Hg). The following variables were considered in the multivariable procedure: age, gender, previous infarction, history of diabetes, history of hypertension, serum creatinine, Killip class on admission, ST-segment elevation infarction, anterior location of infarction, coronary revascularization during hospital course, left ventricular ejection fraction, left atrial dimension, and MR grade.
Receiver operator characteristics curve analysis was used to assess the predictive accuracy of various echocardiographic parameters for the prediction of readmission for HF. Areas under receiver operator characteristics curves were compared using methods described by DeLong et al.
Survival curves were constructed using the Kaplan–Meier method and comparisons were made using the log-rank test. Cox proportional hazard modeling was performed to determine the relation between estimated PASP and the study end points of readmission for treatment of HF and all-cause mortality. Clinical and echocardiographic variables thought to have clinical importance were entered into the model: age, gender, serum creatinine, Killip class at admission, hypertension, diabetes, previous infarction, anterior infarction, ST-segment elevation infarction, coronary revascularization, left ventricular ejection fraction, MR grade, and left atrial size.
The relation between estimated PASP as a continuous variable and HF was also assessed using cubic spline functions. The restricted cubic spline function allowed us to explore nonlinear relations between estimated PASP and HF.
Reclassification of HF risk was evaluated by comparing predicted risk estimates based on multivariable models (including clinical and echocardiographic variables) with and without estimated PASP taking the actual HF events into account. Net reclassification improvement was calculated by summing reclassification improvements for those with an HF event during follow-up and for those without an HF event. For the later analysis, the predicted 1-year HF probabilities were grouped into 3 risk categories of 0% to <5%, 5% to <20%, and ≥20% based on models with and without estimated PASP. Differences were considered statistically significant at a 2-sided p value <0.05. Statistical analyses were performed using STATA 11 (STATA Corp., College Station, Texas).
Results
From July 2001 through June 2009, 2,023 patients were recruited into the study. Patients were excluded because of previous HF (n = 149), missing echocardiographic data (n = 271), and tricuspid regurgitation jets that were not analyzable (n = 549). The remaining 1,054 patients constituted the study population.
Median estimated PASP in the overall study population was 34 mm Hg (interquartile range 28 to 43). PH was frequent in the study population, present in 470 patients (44.6%), and in 151 patients (14.3%) the estimated PASP was >50 mm Hg. Characteristics of study participants are presented in Table 1 according to the presence or absence of PH. Patients with higher estimated PASP were more likely to be older and women, have higher baseline creatinine, and were more likely to have had a previous MI and a history of diabetes and hypertension; they presented with more anterior infarctions and a higher Killip class. Patients with PH had a larger left atrial dimension and a lower left ventricular ejection fraction. Higher estimated PASP was associated with a higher frequency of moderate or severe MR ( Table 1 ).
| Variable | PASP ≤35 mm Hg | PASP >35 mm Hg | p Value |
|---|---|---|---|
| (n = 584) | (n = 470) | ||
| Age (years) | 60 ± 12 | 69 ± 11 | <0.0001 |
| Women | 136 (23%) | 168 (36%) | <0.0001 |
| Serum creatinine (mg/dl) ⁎ | 1.0 ± 0.5 | 1.2 ± 0.8 | <0.0001 |
| Previous myocardial infarction | 118 (20%) | 125 (27%) | 0.01 |
| Diabetes mellitus | 139 (24%) | 166 (33%) | <0.0001 |
| Smoker | 115 (20%) | 84 (18%) | 0.45 |
| Hypertension | 283 (49%) | 295 (63%) | <0.0001 |
| ST-segment elevation infarction | 498 (85%) | 374 (80%) | 0.02 |
| Anterior infarction | 231 (40%) | 230 (49%) | 0.002 |
| Killip class >I on admission | 75 (13%) | 198 (42%) | <0.0001 |
| Coronary revascularization | 316 (54%) | 196 (42%) | <0.0001 |
| Medications | |||
| Antiplatelet agents | 579 (99%) | 451 (96%) | 0.001 |
| Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers | 504 (87%) | 387 (83%) | 0.11 |
| β Blockers | 514 (88%) | 398 (85%) | 0.19 |
| Echocardiographic parameters | |||
| Left atrial diameter (cm) | 4.0 (3.7–4.3) | 4.2 (3.9–4.6) | <0.0001 |
| Ejection fraction (%) | 48 ± 12 | 39 ± 12 | <0.0001 |
| Mild mitral regurgitation | 263 (45%) | 229 (49%) | 0.23 |
| Moderate/severe mitral regurgitation | 39 (7%) | 117 (25%) | <0.0001 |
⁎ To convert from milligrams per deciliter to micromoles per liter, multiply by 88.4.
Median estimated PASP was significantly higher in patients who developed HF than in patients who did not develop HF (43 mm Hg, interquartile range 37 to 50, vs 32 mm Hg, interquartile range 28 to 40, p <0.0001). During the 1-year follow up, 119 patients (11.3%) were readmitted for treatment of HF, with 97 (9.2%) and 22 (2.1%) HF events occurring in patients with and without PH, respectively. Kaplan–Meier curves for the primary end point of readmission for HF showed a marked increase in readmission for HF in patients with an increased estimated PASP ( Figure 1 ) . Cubic spline analysis demonstrated that the probability of HF during the 1-year follow-up increased sharply with an estimated PASP >30 mm Hg, reaching a plateau at ∼50 mm Hg ( Figure 2 ) , although this should be interpreted with caution because of the paucity of data and wide confidence intervals (CIs) at the end of the curve.
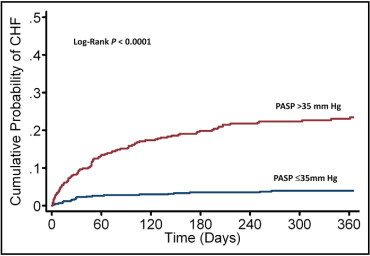
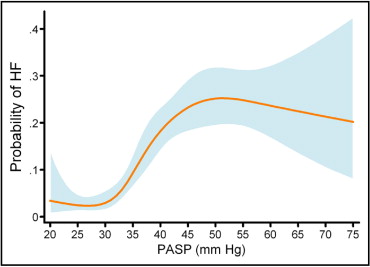
Figure 3 depicts receiver operator characteristics curves for various echocardiographic parameters in the prediction of readmission for HF. Among echocardiographic parameters, PASP displayed the largest area under the curve (0.74 ± 0.02), followed by left ventricular ejection fraction (0.67 ± 0.02), MR grade (0.65 ± 0.02), and left atrial dimension (0.62 ± 0.03). Area under the receiver operator characteristics curve for estimated PASP was significantly larger compared to left atrial dimension (p = 0.0003), left ventricular ejection fraction (p = 0.02), and MR grade (p = 0.001).
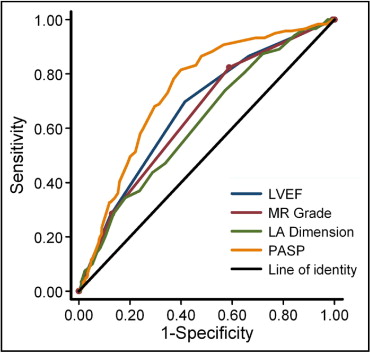
Table 2 presents results of Cox proportional hazards model examining the relation between various parameters including estimated PASP and risk of readmission for HF. On univariable analysis, several clinical and echocardiographic variables were associated with readmission for HF, with a hazard ratio of 5.67 for patients with increased PASP. On multivariable analysis, 6 variables were significantly associated with the primary end point of readmission for HF ( Table 2 ). Increased estimated PASP remained a strong predictor of readmission for HF, with an adjusted hazard ratio of 3.10. Using similar models, the adjusted hazard ratio for increased estimated PASP for the combined end point of HF and death was 1.83 (95% CI 1.31 to 2.57, p <0.0001).
| Variable | Unadjusted HR | 95% CI | p Value | Adjusted HR | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Age (per 10 years) | 1.60 | 1.38–1.85 | <0.0001 | 1.27 | 1.06–1.51 | 0.008 |
| Male gender | 1.70 | 1.21–2.41 | 0.002 | — | — | — |
| Previous infarction | 1.64 | 1.15–2.36 | 0.002 | — | — | — |
| Serum creatinine (mg/dl) | 1.31 | 1.14–1.50 | <0.0001 | — | — | — |
| Diabetes mellitus | 2.08 | 1.48–2.92 | <0.0001 | — | — | — |
| Hypertension | 2.19 | 1.51–3.16 | <0.0001 | 1.51 | 1.00–2.26 | 0.048 |
| Heart rate >100 beats/min | 1.81 | 1.14–2.88 | 0.013 | |||
| Killip class > I | 3.37 | 2.41–4.72 | <0.0001 | 1.76 | 1.20–2.58 | 0.004 |
| Mitral regurgitation | ||||||
| No/trivial | 1.0 (referent) | — | — | 1.0 (referent) | — | — |
| Mild | 2.47 | 1.57–3.90 | <0.0001 | 1.85 | 1.12–3.05 | 0.016 |
| Moderate/severe | 4.87 | 2.94–8.05 | <0.0001 | 2.19 | 1.25–3.86 | 0.007 |
| Left ventricular ejection fraction | ||||||
| ≥45 | 1.0 (referent) | — | — | 1.0 (referent) | — | — |
| 30–45% | 2.92 | 1.96–4.34 | <0.0001 | 1.59 | 1.02–2.48 | 0.041 |
| <30% | 5.17 | 3.92–8.12 | <0.0001 | 2.08 | 1.26–3.44 | 0.004 |
| Pulmonary artery systolic pressure >35 mm Hg | 5.67 | 3.74–8.60 | <0.0001 | 3.10 | 1.87–5.14 | <0.0001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


