The association between anatomic features of the left anterior descending artery (LAD) and outcomes in patients with anterior ST-segment elevation myocardial infarction (STEMI) has not been fully investigated. We sought to clarify the impact of an LAD coronary artery wrapping around the left ventricular (LV) apex on clinical outcomes in patients with anterior STEMI. Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction enrolled patients with STEMI presenting <12 hours after symptom onset who underwent primary percutaneous coronary intervention. Patients with a culprit lesion in the LAD were categorized as (1) LAD wrapping around the LV apex (wrap-around LAD, n = 871) versus (2) LAD not wrapping around the LV apex (non–wrap-around LAD, n = 224). Killip class ≥II, dysrhythmia, and LV mural thrombi were more frequently observed in the wrap-around LAD group; LV ejection fraction was worse in the wrap-around LAD group (54.5% vs 58.7%, p = 0.006). At 3 years of follow-up, major adverse cardiac events (death, stroke, or stent thrombosis, 12.7% vs 5.4%, p = 0.002), death (6.6% vs 3.2%, p = 0.052), stroke (1.9% vs 0.5%, p = 0.12), stent thrombosis (5.6% vs 2.3%, p = 0.047), and severe heart failure (4.5% vs 1.4%, p = 0.03) were more common in patients with a wrap-around LAD versus those with a non–wrap-around LAD. Multivariate analysis indicated that a wrap-around LAD independently and significantly predicted major adverse cardiac events (hazard ratio 2.18, p = 0.02) and severe heart failure (odds ratio 3.31, p = 0.049) in patients with an anterior STEMI. In conclusion, a wrap-around LAD predicted adverse clinical outcomes at 3 years in patients with anterior STEMI who underwent primary percutaneous coronary intervention.
Acute myocardial infarction is the most common cause of mortality in developed countries. Anterior ST-segment elevation myocardial infarction (STEMI), which is caused by left anterior descending artery (LAD) occlusion, is associated with the greatest risk for adverse clinical outcomes because of the extent of jeopardized myocardium. Using data from the INFUSE-AMI (Intracoronary Abciximab and Aspiration Thrombectomy in Patients with Large Anterior Myocardial Infarction) trial, we previously reported that an LAD wrapping around the left ventricular (LV) apex was a predictor for adverse clinical outcomes in a relatively small cohort of patients with anterior STEMI. Therefore, we sought to clarify the impact of LAD wrapping around the LV apex on clinical outcomes in patients with anterior STEMI using the large cohort of patients with 3-year outcome data from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) randomized clinical trial.
Methods
The design and results of HORIZONS-AMI have been described in detail. In brief, HORIZONS-AMI was a prospective, open-label, multicenter, dual-arm, 2 × 2 factorial, randomized trial in patients with STEMI presenting <12 hours after symptom onset undergoing primary percutaneous coronary intervention (PCI). A first randomization was performed in 3,602 patients to the direct thrombin inhibitor bivalirudin alone versus heparin plus a glycoprotein IIb/IIIa inhibitor (1:1 randomization), and then, a second randomization was performed in 3,006 patients to the TAXUS Express paclitaxel-eluting stent versus an equivalent Express bare metal stent (3:1 randomization; Boston Scientific, Natick, Massachusetts). Clinical follow-up was performed at 30 days, 6 months, 1 year, and then annually for 3 years.
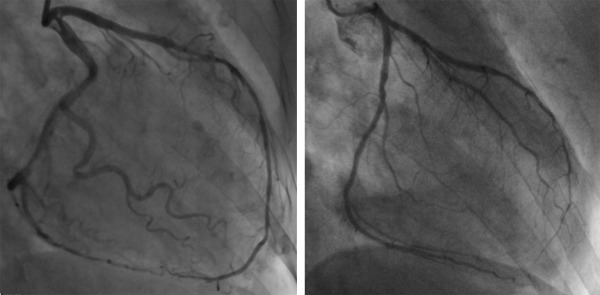
Of the 3,602 patients in HORIZONS-AMI, patients with LAD culprit lesions treated with primary PCI were included in the present substudy. Two independent cardiologists (NK, SJB) who were blinded to the clinical data categorized patients into 2 groups on the basis of coronary angiography as follows: (1) patients with an LAD wrapping around LV apex (wrap-around LAD) and (2) patients with an LAD not wrapping around LV apex (non–wrap-around LAD). Wrap-around LAD was defined as an LAD reaching beyond the LV apex to supply the apical inferior aspect of the heart; non–wrap-around LAD was defined as an LAD terminating at or before the LV apex ( Figure 1 ). Patients who did not obtain Thrombolysis in Myocardial Infarction (TIMI) flow grade >1 during the PCI procedure were excluded from the present study. In addition, all angiograms were reviewed in detail at an independent core laboratory (Cardiovascular Research Foundation, New York, New York) without knowledge of treatment assignment. Specifically, qualitative and quantitative coronary angiography analyses were performed for TIMI flow grade and myocardial blush grade (MBG) at baseline, before stent, and after intervention (final) using standard definitions. MBG was assessed according to the semiquantitative densitometric method that evaluates the maximal intensity of contrast penetrating the infarct zone in comparison to unaffected territories.
The ratio of the peak creatine kinase-MB value within 48 hours from the STEMI onset/local laboratory upper limit of normal was used because multiple commercially available assays for creatine kinase-MB were used in the present multicenter study. LV mural thrombi detected by echocardiography, left ventriculography, cardiac magnetic resonance imaging, or cardiac computed tomography were reported by each site. Adverse clinical events including death, reinfarction, stroke, and stent thrombosis were adjudicated by a Clinical Events Committee blinded to patient allocation. Severe heart failure, defined as the New York Heart Association classes III or IV, was site reported but not centrally adjudicated. Diagnosis of stent thrombosis was based on the Academic Research Consortium criteria. Major adverse cardiac events (MACE) were defined as death, stroke, or culprit lesion–related definite or probable stent thrombosis.
Baseline characteristics and clinical outcomes were analyzed on a patient level, whereas angiographic characteristics were analyzed on a lesion level. Categorical variables are summarized as frequencies and compared between groups using the chi-square or Fisher’s exact test. Continuous variables are displayed as median and interquartile range and compared using the Wilcoxon rank-sum statistics. Time-to-event outcomes including MACE, death, stroke, and stent thrombosis are summarized as Kaplan–Meier estimates of cumulative incidence and compared between groups with the log-rank test. Because the onset date of heart failure was not available, heart failure was evaluated as a binary variable (i.e., not time dependent). Cox proportional hazards regression models were conducted to determine the independent predictors for MACE, death, stroke, and stent thrombosis. Candidates for independent predictors for Cox proportional hazard models included wrap-around LAD, age, diabetes mellitus, history of myocardial infarction, dysrhythmia, 3-vessel disease with left main disease, proximal LAD culprit lesion location, final TIMI flow grade <3, total length of implanted stents, final lesion minimum lumen diameter, paclitaxel-eluting stent use, bivalirudin use, clopidogrel 600-mg loading dose, and statin use at discharge. To determine the independent predictors for severe heart failure, multiple logistic regression analysis was conducted including wrap-around LAD, age, diabetes mellitus, history of myocardial infarction, and proximal LAD culprit lesion location. Intraobserver and interobserver variability for the diagnosis of a wrap-around LAD was measured with the κ test of concordance. Statistical significance was set at p <0.05. Statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, North Carolina).
Results
Of the 3,602 patients enrolled in HORIZONS-AMI, 193 patients were treated medically and 64 patients had bypass surgery or delayed PCI. Thus, primary PCI was performed in 3,345 patients. Of them, 1,276 patients with a culprit lesion in the LAD were enrolled in the present substudy and categorized as wrap-around LAD or non–wrap-around LAD. Because of poor quality of angiograms or postprocedure TIMI flow grade 0 to 1, in which it was not possible to assess termination of the LAD, 181 patients (14.2%) could not be classified. Of the remaining 1,095 patients, 871 patients (79.5%) had a wrap-around LAD, and 224 patients (20.5%) had a non–wrap-around LAD. Interobserver (NK vs SJB) and intraobserver (NK, baseline vs 4 months later) variabilities in classification were 0.84 and 0.84, respectively.
Baseline clinical features, in patients with versus without a wrap-around LAD, are provided in Table 1 . The prevalence of hypertension, Killip class ≥II, and major dysrhythmia were higher in patients with a wrap-around LAD than in those with a non–wrap-around LAD. LV mural thrombi tended to be more frequently observed in patients with a wrap-around LAD than in those with a non–wrap-around LAD; as a result, there was a similar trend in the frequency of warfarin use at discharge.
| Variable | Wrap-around LAD | p Value | |
|---|---|---|---|
| Yes (n=871) | No (n=224) | ||
| Age (years) | 60.3 (52.8–70.4) | 59.1 (51.5–69.9) | 0.31 |
| Men | 78.1% (680/871) | 76.3% (171/224) | 0.58 |
| Body mass index (kg/m 2 ) | 26.9 (24.2–30.1) | 27.8 (25.0–30.7) | 0.02 |
| Hypertension | 53.3% (463/869) | 45.5% (102/224) | 0.04 |
| Hyperlipidemia | 39.5% (343/869) | 39.3% (88/224) | 0.96 |
| Diabetes mellitus | 17.8% (155/869) | 13.4% (30/224) | 0.11 |
| Current smoker | 56.6% (491/867) | 57.6% (129/224) | 0.80 |
| Renal insufficiency ∗ | 17.1% (137/802) | 13.7% (28/204) | 0.25 |
| Prior myocardial infarction | 8.2% (71/869) | 5.8% (13/224) | 0.24 |
| Prior percutaneous coronary intervention | 8.4% (73/869) | 9.8% (22/224) | 0.50 |
| Prior coronary artery bypass grafting | 0.7% (6/869) | 0% (0/224) | 0.36 |
| Killip classification | |||
| I | 86.5% (750/867) | 92.4% (207/224) | 0.02 |
| II | 10.8% (94/867) | 7.1% (16/224) | 0.10 |
| III | 1.4% (12/867) | 0% (0/224) | 0.14 |
| IV | 1.3% (11/867) | 0.4% (1/224) | 0.48 |
| Major dysrhythmia | 4.4% (38/869) | 1.3% (3/224) | 0.03 |
| Peak creatine kinase-MB/upper limit of normal | 16.6 (6.2–37.4) | 13.9 (5.8–34.9) | 0.34 |
| Left ventricular mural thrombi | 2.3% (20/869) | 0.4% (1/224) | 0.10 |
| Randomized to bivalirudin use | 46.7% (407/871) | 52.7% (118/224) | 0.12 |
| Clopidogrel 600 mg loading | 61.9% (529/855) | 62.9% (139/221) | 0.78 |
| Medication at the discharge | |||
| Beta-blockers | 90.6% (782/863) | 95.5% (214/224) | 0.02 |
| ACE Inhibitor or ARB | 88.4% (754/853) | 89.7% (200/223) | 0.59 |
| Statin | 95.9% (818/853) | 95.1% (212/223) | 0.59 |
| Warfarin | 6.4% (55/853) | 3.6% (8/223) | 0.11 |
∗ Renal insufficiency was defined as a creatinine clearance <60 ml/min as calculated with the use of the Cockcroft–Gault equation.
Angiographic lesion findings are provided in Table 2 . Preprocedural lesion length and postprocedure stent length were longer in lesions with versus without a wrap-around LAD. Preprocedural and postprocedural TIMI flow grade and postprocedural MBG were comparable between the 2 groups. Angiographic LV ejection fraction was significantly lower in patients with a wrap-around LAD than in those with a non–wrap-around LAD.
| Variable | Wrap-around LAD | p Value | |
|---|---|---|---|
| Yes (n=871) | No (n=224) | ||
| Symptom onset to balloon, min | 213 (160–330) | 224 (160–335) | 0.59 |
| Randomized to paclitaxel-eluting stent use | 73.7% (600/814) | 78.2% (161/206) | 0.19 |
| Total length of implanted stents (mm) | 24.0 (18.0–36.0) | 21.5 (16.0–32.0) | 0.007 |
| Pre-procedure | 925 lesions | 230 lesions | |
| Multi vessel disease ∗ | 76.8% (668/870) | 71.0% (159/224) | 0.07 |
| Proximal LAD culprit lesion | 32.1% (297/925) | 33.5% (77/230) | 0.69 |
| ACC/AHA classification type B 2 /C | 87.0% (805/925) | 85.7% (197/230) | 0.58 |
| Lesion length (mm) | 15.00 (10.42–20.00) | 13.31 (10.00–18.86) | 0.02 |
| Reference vessel diameter (mm) | 2.79 (2.49–3.08) | 2.74 (2.42–2.99) | 0.08 |
| Minimum lumen diameter (mm) | 0.09 (0.00–0.62) | 0 (0.00–0.52) | 0.14 |
| TIMI flow grade | |||
| 0 or 1 | 58.5% (506/865) | 61.6% (138/224) | 0.40 |
| 2 | 17.9% (155/865) | 16.1% (36/224) | 0.52 |
| 3 | 23.6% (204/865) | 22.3% (50/224) | 0.69 |
| Thrombus | 13.5% (490/3640) | 14.6% (132/905) | 0.38 |
| Post-procedure | |||
| In-segment minimum lumen diameter (mm) | 2.19 (1.94–2.51) | 2.18 (1.88–2.44) | 0.13 |
| TIMI flow grade | |||
| 0 or 1 | 1.3% (11/865) | 1.8% (4/224) | 0.53 |
| 2 | 14.3% (124/865) | 12.9% (29/224) | 0.59 |
| 3 | 84.4% (730/865) | 85.3% (191/224) | 0.75 |
| Myocardial blush grade | |||
| 0 or 1 | 23.7% (206/870) | 25.1% (56/223) | 0.65 |
| 2 or 3 | 76.3% (664/870) | 74.9% (167/223) | |
| Ejection fraction on left ventriculography (%) | 54.50 (43.90–63.90) | 58.70 (48.70–65.90) | 0.006 |
∗ Patients with ≥2 diseased vessels (diameter stenosis ≥30%).
Long-term outcomes through 3 years are provided in Table 3 and Figure 2 . The Kaplan–Meier estimate cumulative incidence of MACE (p = 0.002), death (p = 0.052), stroke (p = 0.12), and stent thrombosis (p = 0.047) were greater in patients with a wrap-around LAD than in those with a non–wrap-around LAD. The frequency of heart failure was significantly higher in patients with a wrap-around LAD than in those with a non–wrap-around LAD (p = 0.03).
| Variable | Wrap-around LAD (n = 871) | Non–wrap-around LAD (n = 224) | Risk ratio | p Value |
|---|---|---|---|---|
| Major adverse cardiac events # | 12.7% (108) ∗ | 5.4% (12) ∗ | 2.45 (1.35–4.44) † | 0.002 ‡ |
| Death | 6.6% (56) ∗ | 3.2% (7) ∗ | 2.14 (0.97–4.69) † | 0.052 ‡ |
| Stroke | 1.9% (16) ∗ | 0.5% (1) ∗ | 4.31 (0.57–32.49) † | 0.12 ‡ |
| Stent thrombosis | 5.6% (46) ∗ | 2.3% (5) ∗ | 2.46 (0.98–6.20) † | 0.047 ‡ |
| Heart failure (NYHA class III/IV) | 4.5% (38/846) § | 1.4% (3/220) § | 3.29 (1.03–10.57) ¶ | 0.03 ‖ |
∗ Kaplan–Meier estimates cumulative incidences (number of events).
† Hazard Ratio (95% confidence interval).
§ Non-cumulative incidence (event/total).
¶ Odds ratio (95% confidence interval).
# Death, stroke, or culprit lesion-related definite or probable stent thrombosis.
Multivariate Cox proportional hazard analysis showed that a wrap-around LAD independently and significantly predicted MACE ( Table 4 ). Multivariate logistic regression analysis also indicated that a wrap-around LAD significantly predicted severe heart failure ( Table 5 ) and independent of lesion location in the LAD (proximal vs mid vessel).
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p Value | Hazard Ratio | 95% Confidence Interval | p Value | |
| To predict MACE | ||||||
| Wrap-around LAD | 2.50 | 1.44–4.33 | 0.001 | 2.18 | 1.16–4.09 | 0.02 |
| Age (10-year increase) | 1.04 | 1.03–1.04 | <0.0001 | 1.17 | 0.99–1.38 | 0.07 |
| Diabetes mellitus | 1.64 | 1.33–2.02 | <0.0001 | |||
| History of myocardial infarction | 1.76 | 1.39–2.22 | <0.0001 | 2.11 | 1.23–3.63 | 0.007 |
| Major dysrhythmia | 1.73 | 1.15–2.61 | 0.009 | 2.43 | 1.28–4.65 | 0.007 |
| 3 Vessel with left main disease | 2.40 | 1.48–3.89 | 0.0004 | |||
| Final TIMI flow grade 3 | 0.57 | 0.45, 0.72 | <0.0001 | |||
| Proximal LAD culprit lesion | 1.26 | 0.97–1.63 | 0.09 | |||
| Paclitaxel-eluting stent use | 1.01 | 0.80-1.27 | 0.93 | |||
| Bivalirudin use | 0.87 | 0.73-1.04 | 0.14 | |||
| Final lesion minimum lumen diameter (1-mm increase) | 0.73 | 0.64–0.85 | <0.0001 | |||
| Statin use at discharge | 0.45 | 0.32–0.62 | <0.0001 | 0.36 | 0.19–0.68 | 0.002 |
| To predict death | ||||||
| Wrap-around LAD | 2.31 | 1.11–4.80 | 0.02 | 1.68 | 0.71–3.97 | 0.24 |
| Age (10-year increase) | 1.08 | 1.07–1.09 | <0.0001 | 1.74 | 1.33–2.28 | <0.0001 |
| Major cardiac rhythm or rate disturbance | 2.36 | 1.48–3.75 | 0.0003 | 3.83 | 1.74–8.39 | 0.001 |
| 3 Vessel with left main disease | 3.64 | 2.16–6.12 | <0.0001 | |||
| Final TIMI flow grade 3 | 0.47 | 0.35 – 0.62 | <0.0001 | |||
| Statin use at discharge | 0.31 | 0.20–0.46 | <0.0001 | 0.39 | 0.16–0.93 | 0.04 |
| To predict stroke | ||||||
| Wrap-around LAD | 2.37 | 0.55–10.21 | 0.25 | 4.11 | 0.54–31.00 | 0.17 |
| Age (10-year increase) | 1.04 | 1.02–1.06 | 0.0004 | |||
| History of myocardial infarction | 2.02 | 1.13–3.63 | 0.02 | 3.76 | 1.23–11.54 | 0.02 |
| To predict stent thrombosis | ||||||
| Wrap-around LAD | 2.35 | 1.01–5.46 | 0.047 | 2.35 | 0.93–5.93 | 0.07 |
| History of myocardial infarction | 1.36 | 0.88–2.08 | 0.16 | 2.32 | 1.09–4.94 | 0.03 |
| Total length of implanted stents | 1.01 | 1.00–1.02 | 0.003 | |||
| Final lesion minimum lumen diameter (1-mm increase) | 0.80 | 0.62–1.03 | 0.08 | |||
| Clopidogrel 600 mg loading dose | 0.70 | 0.53–0.94 | 0.02 | 0.54 | 0.31–0.93 | 0.03 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


