The safety and efficacy of an empiric superior vena cava isolation (SVCI) in addition to circumferential pulmonary vein isolation (CPVI) in patients with paroxysmal atrial fibrillation (PAF) have not been clarified. A total of 186 consecutive patients who underwent catheter ablation of PAF were included. All patients underwent a CPVI. Patients in the first half underwent an additional SVCI only if SVC-triggered AF or rapid SVC activity was observed during the procedure (n = 93, as-needed SVCI, group I), and those in the second half underwent an empirical SVCI after the CPVI (n = 93, empiric SVCI, group II). The CPVI was successfully performed in all patients. An SVCI was performed in 8 of 93 patients (9%) in group I and 81 of the 93 patients (87%) in group II. In the remaining 12 patients in group II, an SVCI was not performed because of the lack of SVC potentials. During a mean follow-up of 27 ± 12 months, the atrial tachyarrhythmia recurrence rate after a single ablation procedure in the patients in group II was lower than that in group I (44% vs 23%, p = 0.035). A Cox regression multivariate analysis demonstrated that an empiric SVCI was an independent predictor of an atrial tachyarrhythmia recurrence after a single ablation procedure (odds ratio: 0.57, 95% confidence interval 0.31 to 0.999; p = 0.049). Neither sinus node injury nor any injury to the phrenic nerve was observed. In conclusion, an empiric SVCI in addition to the CPVI improved the outcome of AF ablation in patients with PAF without any additional adverse effects.
Because it was reported that paroxysmal atrial fibrillation (PAF) is most often triggered by sources inside the pulmonary veins (PVs), ablation strategies that target the PVs and/or PV antrum are the cornerstone for most AF ablation procedures. The empiric isolation of all PVs is a generally accepted strategy for the ablation of AF whether each PV is arrhythmogenic. The superior vena cava (SVC) has been established as one of the important sources of AF, and the electric isolation is the standard ablation strategy for an arrhythmogenic SVC. However, whether an empiric SVC isolation (SVCI) in addition to the circumferential PV isolation (CPVI) improves the outcome of AF ablation in patients with PAF is still controversial. The aim of this study was to compare the procedural safety and outcome between an as-needed SVCI and empiric SVCI in addition to the CPVI.
Methods
From February 2011 to June 2014, 186 consecutive patients without any moderate or severe left ventricular systolic dysfunction (left ventricular ejection fraction ≥40%) who underwent catheter ablation of symptomatic PAF for the first time were included in this retrospective study. All patients underwent a CPVI. Ninety-three patients underwent an additional SVCI only if SVC-triggered AF or rapid SVC activity was observed during the procedure (as-needed SVCI, group I) before January 2013, and 93 patients empirically underwent an SVCI after the CPVI ( empiric SVCI, group II) after February 2013. PAF was defined according to the Heart Rhythm Society, the European Society of Cardiology, and the European Cardiac Arrhythmia Society 2012 Consensus Statement on Catheter and Surgical Ablation of AF. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. It was approved by the Institutional Review Board and the Ethical Committee of the Tokyo Women’s Medical University. A written informed consent was obtained from all patients.
As a preoperative evaluation, all patients underwent transthoracic echocardiography, transesophageal echocardiography, and multidetector computed tomography using a 64-slice computed tomography scanner within 2 days before the scheduled ablation procedure. All echocardiographic parameters were measured according to the recommendations of the American Society of Echocardiography. The multidetector computed tomography image was obtained with contrast enhancement in most patients but without contrast enhancement in those with renal dysfunction. All patients were effectively anticoagulated for >1 month, and transesophageal echocardiography was performed to exclude any left atrium (LA) thrombi before the ablation procedure. Before the procedure, all antiarrhythmic drugs (AADs) were discontinued for at least 5 half-lives except for amiodarone. Anticoagulation therapy using warfarin with a therapeutic international normalized ratio was continued during the periprocedural period.
Using a jugular or femoral venous approach, a decapolar catheter was placed in the coronary sinus. Using a femoral venous approach, 2 or 3 long sheaths were introduced into the LA, and a single transseptal puncture was performed under intracardiac echocardiographic guidance. After the transseptal puncture an initial intravenous bolus of 5,000 IU of heparin was given, and repeated doses of heparin were given to maintain an activated clotting time between 300 and 350 seconds. All patients underwent a wide CPVI guided by electroanatomic mapping combined with image integration. A 3.5-mm cooled-tip catheter (Navistar ThermoCool or ThermoCool SF; Biosense Webster Inc., California) was used for mapping and ablation. The circumferential ablation lines were created approximately 1 to 3 cm from the ipsilateral PV ostia. PV isolation was defined as bidirectional block between the LA and inside the CPVI area and confirmed by the electrograms recorded from circular mapping catheters (1 duo-decapolar variable radius circular catheter [Lasso; Biosense Webster, Baldwin Park, California], or Optima [St Jude Medical, Minneapolis, Minnesota]) with or without 1 decapolar circular catheter and pacing maneuvers. Radio frequency (RF) energy was delivered to the atrial tissue with a power of 25 to 30 W using irrigation rates of 17 ml/min with the Navistar ThermoCool or 8 ml/min with the Navistar ThermoCool SF to achieve the desired power delivery. The power was limited to 20 to 25 W at the posterior wall of the LA. The temperature was limited to 42°C. After an intravenous injection of a 10 μg bolus of isoproterenol, an administration of 20 mg of adenosine triphosphate (ATP) was given to provoke a reconnection of the PVs (dormant PV conduction) at least 20 minutes after a successful wide CPVI. If any dormant PV conduction was observed, additional RF energy was applied at the earliest PV activation site identified using the circular mapping catheters on the circumferential ablation lines, until the loss of any dormant PV conduction occurred. We targeted the ablation of any atrial tachyarrhythmias (ATAs) induced by programmed electrical stimuli with or without an injection of a 10-μg bolus of isoproterenol.
For an electrical SVCI, the RF was delivered just below the circular mapping catheter, which was placed 5 to 10 mm above the right atrium (RA)-SVC junction guided by electroanatomic mapping combined with image integration ( Figure 1 ), and in a point-by-point fashion using a maximum power of 25 W. Before the RF delivery, high output pacing at 10 mA was performed at a site on the posterior to lateral wall of the SVC, and if diaphragmatic stimulation was observed, ablation at that site was avoided. If the RA-SVC conduction remained at the site of diaphragmatic stimulation, the RF was delivered very carefully while monitoring the diaphragmatic movement on fluoroscopy using a power of 15 to 20 W, for up to 20 seconds. The end point of the SVCI was the elimination of all SVC potentials recorded by the circular catheter.
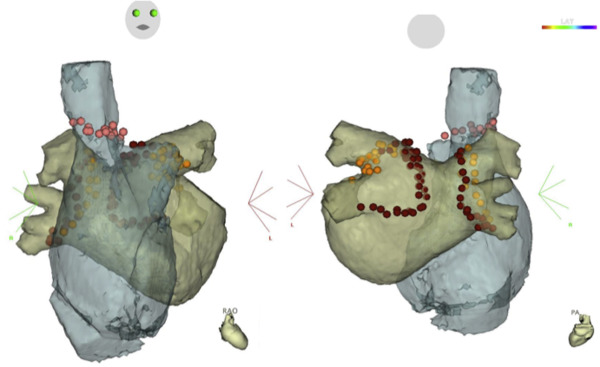
After the CPVI, non-PV triggers were mapped by multielectrode catheters positioned in the coronary sinus, SVC, and RA free wall during an intravenous injection of a 10-μg bolus of isoproterenol with and without a 20-mg bolus injection of ATP. The SVCI was performed only if SVC-triggered AF or rapid SVC activity was observed in group I. The SVCI was systematically and empirically performed after the CPVI, before the provocation of non-PV triggers and dormant PV conduction, in all the patients in group II.
A chest x-ray was undertaken in a standing position before and the next day after the ablation procedure in all patients. Ineffective AADs before the ablation were prescribed only if any early recurrences of an ATA were observed before discharge and discontinued by 3 months after the procedure. All patients were scheduled for visits in the outpatient clinic at 1, 2, 3, 6, 9, and 12 months after the procedure and then every 6 months. The presence of ATAs was evaluated by the patient symptoms, electrocardiographic recordings, and 24-hour ambulatory monitoring (1, 3, 6, 9, and 12 months after the ablation and then every 6 months). Patients with palpitations were encouraged to use portable electrocardiographic monitoring (HCG-801R; Omron, Kyoto, Japan). Recurrence was defined as recurrent symptoms and/or documented ATAs on the electrocardiogram, 24-hour ambulatory monitoring, portable electrocardiographic monitoring, or data provided by cardiac implantable electrical devices (lasting >30 seconds) after a 2-month blanking period from the ablation procedure without any AADs. In the absence of any ATA recurrences after 3 months, the anticoagulant treatment was discontinued unless the patients had a CHADS 2 score of ≥2.
Data were presented as the mean ± standard deviation, percentage, or number, as appropriate. Differences between the continuous values were assessed using an unpaired 2-tailed t test for normally distributed continuous variables, the Mann–Whitney test for skewed variables, and the chi-square test (with Fisher’s exact test) for nominal variables. A Cox proportional hazards model was used to identify any predictors of ATA recurrences after a single ablation procedure. All preprocedural potential confounders were entered into the model on the basis of their known clinical relevance or a significant association being observed in the univariate analysis. All parameters with a significance of <0.05 in the univariate analysis were entered into the multivariate model. A Kaplan–Meier analysis with a log-rank test was used to determine the probability of the freedom from recurrent ATAs after the initial and last procedure. A p value <0.05 was considered statistically significant. All statistical analyses were performed with JMP Pro software version 11.2.0 (SAS Institute, Cary, North Carolina).
Results
The baseline characteristics of the patients of each group are provided in Table 1 . There were no significant differences in the baseline characteristics between the 2 groups.
| Variable | SVC isolation | p value | |
|---|---|---|---|
| As-needed (n=93) | Empiric (n=81) | ||
| Age (years) | 58±12 | 60±10 | 0.52 |
| Male | 66 (71%) | 61 (75%) | 0.52 |
| Body mass index (kg/m 2 ) | 23±3 | 24±3 | 0.76 |
| CHADS2 score | 1±1 | 1±1 | 0.39 |
| CHA2DS2-VAS C score | 2±1 | 1±1 | 0.62 |
| Estimated glomerular filtration rate (mL /min/1.73m 2 ) | 71±19 | 67±14 | 0.14 |
| Left atrial diameter (mm) | 36±6 | 36±5 | 0.9 |
| Left atrial volume (mL) | 52±16 | 51±18 | 0.53 |
| Left atrial volume index (mL/m 2 ) | 30±9 | 30±10 | 0.42 |
| Left ventricular ejection fraction (%) | 55±5 | 56±6 | 0.051 |
| Hypertension | 40 (43%) | 32 (40%) | 0.64 |
| Diabetes mellitus | 12 (13%) | 7 (9%) | 0.37 |
| Hyperlipidemia | 33 (35%) | 25 (31%) | 0.52 |
| Structural heart disease | 11 (12%) | 6 (7%) | 0.33 |
| Coronary artery disease | 1 | 2 | |
| Hypertrophic cardiomyopathy | 3 | 3 | |
| Atrial septum defect | 3 | 0 | |
| Post valve replacement surgery | 2 | 1 | |
| Cardiomyopathy | 2 | 0 | |
| Cardiac implantable electronic device | 4 (4%) | 3 (4%) | 0.84 |
The CPVI was successfully performed in all patients. In the patients in group I, an SVCI was performed in 8 of 93 patients (9%), because of SVC-triggering AF in 3 patients (those were identified by an ATP injection after an isoproterenol injection) and rapid SVC activity in 5 patients (1 spontaneous, 3 with an isoproterenol injection, and 1 with an ATP injection after an isoproterenol injection). During a mean follow-up of 36.3 ± 6.9 months after a single ablation, 41 patients (44%) had an ATA recurrence, including persistent atrial tachycardia (AT) in 3 patients and PAF in 38 patients.
An empiric SVCI could be performed in 81 of 93 patients (87%) in group II. In the remaining 12 patients, no SVCI was performed because of the lack of SVC potentials. The mean procedure time for the SVCI was comparable between the 2 groups ( Table 2 ). Dissociated potentials were revealed in the isolated area of the SVC in 25 of 81 patients (31%) in group II. During a mean follow-up of 15.7 ± 5.1 months after a single ablation procedure, 19 patients (23%) in group II had an ATA recurrence, including persistent AT in 3 patients, persistent common atrial flutter in 1 patient, and PAF in 15 patients. There was no significant difference in the ratio of the patients who underwent portable electrocardiographic monitoring (16 patients [18%] vs 12 patients [15%], p = 0.67).
| Variable | SVC isolation | p value | |
|---|---|---|---|
| As-needed (n=93) | Empiric (n=81) | ||
| Total procedural time (min) | 208.7±54.0 | 181.0±58.3 | 0.0001 |
| Superior vena cava isolation time (min) | 9.7±4.4 | 7.2±4.9 | 0.10 |
| Fluoroscopic time (min) | 30.2±15.1 | 16.7±7.3 | <0.0001 |
| Complications | 1 (1.1%) | 3 (3.7%) | 0.6 |
| SVC isolation | 8 (9%) | 81 (100%) | < 0.0001 |
| Cavotricuspid isthmus linear ablation | 24 (26%) | 19 (23%) | 0.72 |
| Other ablation targets | 8 (9%) | 4 (5%) | 0.39 |
| Frequent atrial premature contractions | 2 | 0 | |
| Atrial tachycardia | 5 | 3 | |
| Wolf-Parkinson-White syndrome | 1 | 0 | |
| Atrioventricular nodal reentrant tachycardia | 1 | 0 | |
| Frequent ventricular premature contractions | 0 | 1 | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


