Guidelines recommend screening cardiovascular magnetic resonance (Sc-CMR) imaging for all patients after coarctation of the aorta repair, although there are limited data verifying its clinical utility. Therefore, we sought to assess the value of Sc-CMR in detecting aortic complications and at-risk abnormalities after coarctation of the aorta repair and to identify significant risk factors. We reviewed 76 patients (mean age 31 ± 10 years), including 40 with symptomatically indicated CMR (Sx-CMR) and 36 with Sc-CMR studies. CMR angiograms were evaluated for aortic abnormalities. Recoarctation was defined as residual narrowing/descending aorta at the diaphragm ≤0.5 (at risk ≤0.75), ascending aorta aneurysm as maximum ascending cross-sectional area/height ≥10 (at risk ≥5), and descending aorta aneurysm as maximum descending diameter/descending aorta at the diaphragm ≥1.5 (at risk ≥1.25). Aortic complications or abnormalities were found in 45 patients (59%). No patient met criteria for recoarctation (at risk 10 Sx-CMR vs 5 Sc-CMR). Significant risk factors included heart failure symptoms and female gender (p <0.05). One patient (Sc-CMR) had ascending aneurysm (at risk 17 Sx-CMR vs 8 Sc-CMR). Time from repair was a significant predictor (p <0.05). There were 10 patients (6 Sx-CMR vs 4 Sc-CMR) with descending aneurysm (at risk 8 Sx-CMR vs 7 Sc-CMR). Cardiovascular symptoms, hypertension, and echocardiogram were not predictive. In conclusion, >50% of patients undergoing Sc-CMR had aortic abnormalities, which was not significantly different from those undergoing Sx-CMR. In particular, Sc-CMR identified descending aorta aneurysms that were not predicted by clinical parameters or echocardiogram.
The American College of Cardiology/American Heart Association practice guidelines recommend that every patient with repaired coarctation of the aorta (CoA) should have a screening cardiovascular magnetic resonance (Sc-CMR) imaging study to evaluate the thoracic aorta for abnormalities, regardless of clinical findings. However, there are limited data to support this approach and its utility in clinical practice. Therefore, we sought to determine in a retrospective review the frequency of aortic complications detected by CMR in patients with repaired CoA undergoing screening assessment. Our secondary objectives were to characterize patients with at-risk aortic abnormalities detected by symptomatically indicated CMR (Sx-CMR) or Sc-CMR study who might benefit from increased surveillance and to identify significant risk factors.
Methods
This study was reviewed and approved by The Ohio State University institutional review board. Patients included all adolescents and adults ≥13 years of age who underwent CMR evaluation for previously repaired CoA at The Ohio State University institution from 2003 to 2009. Patients with single-ventricle anatomy or multiple left-sided obstructive lesions (e.g., Shone complex) were excluded. Studies were also excluded if the primary indication was evaluation of the thoracic aorta after recent (<1 year) surgical or transcatheter intervention.
Details on demographic variables, anthropometrics, CoA repair history, cardiovascular symptoms, upper and lower extremity systolic blood pressures at rest, echocardiographic results, and CMR findings were collected. Associated bicuspid aortic valve was noted. Original CoA repairs included resection with end-to-end anastomosis, prosthetic patch aortoplasty graft, subclavian flap aortoplasty, interposition tube graft, and primary transcatheter balloon angioplasty.
Patients were considered to have Sx-CMR studies if they had systemic hypertension (arm systolic blood pressure ≥140 mm Hg), upper-to-lower extremity gradient (≥20 mm Hg), cardiovascular symptoms, or evidence of recoarctation or left ventricular systolic dysfunction (ejection fraction <50%) by echocardiogram. The remaining studies were considered Sc-CMR.
CMR examinations were performed on a 1.5-T MR scanner (Magnetom Avanto, Siemens Medical Solutions, Inc., Malvern, Pennsylvania). Contrast-enhanced 3-dimensional angiographic data were acquired using a heavily T1-weighted spoiled gradient echocardiographic sequence obtained in the coronal plane. Contrast angiography was performed using a traditional timing bolus technique. We recorded CMR left ventricular measurements, including volumes and mass indexed for body surface area, and ejection fraction. Measurements of the thoracic aorta were determined at multiple levels by 3-dimensional CMR angiography ( Figure 1 ). All measurements were indexed to the descending aorta at the level of the diaphragm and using a ratio of aortic cross-sectional area to height.
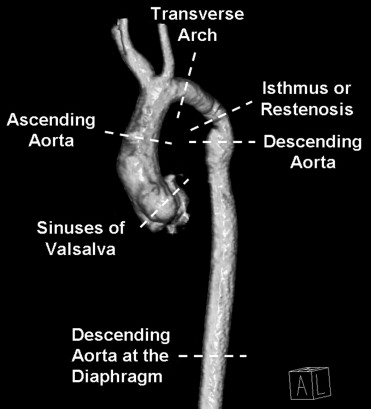
We defined ascending and descending thoracic aorta complications based on previously reported studies and at-risk aortic abnormalities using modified criteria. Recoarctation was defined as diameter of restenosis or residual narrowing/diameter of descending aorta at the diaphragm ≤0.5 and at-risk recoarctation as 0.5 to 0.75. Ascending aorta aneurysm was defined as maximum ascending aorta diameter with cross-sectional area (square centimeters)/height (meters) ≥10 and at-risk ascending aneurysm as 5 to 10. Descending aorta aneurysm was defined as maximum descending aorta diameter/diameter of descending aorta at the diaphragm ≥1.5 and at-risk descending aneurysm as 1.25 to 1.5.
Continuous data are presented as mean ± SD. Patients with Sx-CMR were compared to those undergoing Sc-CMR using Student’s t test. Chi-square tests were used to compare categorical variables. Ordinary least-squares linear regression and logistic regression were used to identify risk factors that predicted aortic abnormalities. Two-tailed p values <0.05 were considered statistically significant.
Results
In total 76 patients underwent CMR for CoA evaluation, and baseline characteristics are listed in Table 1 . The cohort consisted of 43 men (57%) and 33 women (43%), with a mean age at CMR of 31 ± 11 years. Initial repairs included 32 end-to-end anastomoses (42%), 15 patch aortoplasties (20%), 12 subclavian flap aortoplasties (16%), 3 primary balloon angioplasties (4%), and 1 interposition tube graft (1%). Operative reports were unavailable for 13 patients (17%). Mean age at repair was 7 ± 7 years (range 0.01 to 36). Time from initial repair to CMR was 24 ± 10 years (range 1 to 55).
| Variable | Sx-CMR | Sc-CMR | p Value |
|---|---|---|---|
| (n = 40) | (n = 36) | ||
| Women | 14 (35%) | 19 (53%) | NS |
| Height (m) | 1.7 ± 0.1 | 1.7 ± 0.1 | NS |
| Weight (kg) | 86 ± 23 | 78 ± 17 | NS |
| Body surface area (m 2 ) | 2.0 ± 0.3 | 1.9 ± 0.2 | NS |
| Bicuspid aortic valve | 22 (55%) | 15 (42%) | NS |
| Age at repair (years) | 6 ± 7 | 7 ± 7 | NS |
| Age at magnetic resonance (years) | 30 ± 9 | 31 ± 12 | NS |
| Time from repair to magnetic resonance (years) | 24 ± 8 | 23 ± 12 | NS |
| Arm systolic blood pressure (mm Hg) | 137 ± 16 | 119 ± 10 | <0.001 |
| Hypertension | 24 (60%) | 0 (0%) | <0.001 |
| Angina pectoris | 10 (25%) | 0 (0%) | <0.01 |
| New York Heart Association functional class ≥II | 5 (13%) | 0 (0%) | <0.05 |
| Echocardiogram: recoarctation | 11/38 (29%) | 0/29 (0%) | <0.01 |
| Echocardiogram: left ventricular ejection fraction <50% | 9/38 (24%) | 0/29 (0%) | <0.01 |
Bicuspid aortic valve was present in 37 patients (49%). Mean upper extremity blood pressure was 129 ± 16 mm Hg. Systemic hypertension was present in 24 patients (32%). Upper and lower extremity blood pressures were available for comparison in 56 patients, and only 1 had documented upper-to-lower extremity blood pressure gradient >20 mm Hg. Cardiovascular symptoms included chest discomfort in 13% and heart failure symptoms in 7%.
Echocardiogram was obtained in 88% of patients before CMR. Suspected recoarctation was based on moderate to severe narrowing at the site of previous coarctation repair or peak-to-peak gradient ≥20 mm Hg with Doppler imaging and was found in 16% (n = 11) of studies. Aortic root dilation was noted in 13% (n = 9), but descending aorta aneurysm was not reported in any study. Left ventricular systolic dysfunction was present in 9 patients (13%).
There were 40 patients undergoing Sx-CMR based on clinical or echocardiographic abnormalities as previously defined. Therefore, 36 patients underwent Sc-CMR. There were no significant differences between these groups in gender, anthropometrics, type of surgical repair, age at repair, age at CMR, or time from surgery to CMR ( Table 1 ). CMR findings were similar in the 2 groups, with no significant differences in left ventricular ejection fraction or indexed end-diastolic and end-systolic volumes. Left ventricular mass was significantly increased in patients undergoing Sx-CMR ( Table 2 ).
| Variable | Sx-CMR | Sc-CMR | p Value |
|---|---|---|---|
| (n = 40) | (n = 36) | ||
| Left ventricular end-diastolic volume (ml/m 2 ) | 67 ± 18 | 61 ± 16 | NS |
| Left ventricular end-systolic volume (ml/m 2 ) | 28 ± 12 | 25 ± 10 | NS |
| Left ventricular ejection fraction (%) | 60 ± 10 | 61 ± 8 | NS |
| Left ventricular mass (gm/m 2 ) | 64 ± 22 | 53 ± 14 | <0.05 |
| Recoarctation | 0 (0%) | 0 (0%) | |
| At-risk recoarctation | 10 (25%) | 5 (14%) | NS ⁎ |
| Ascending aortic aneurysm | 0 (0%) | 1 (3%) | NS ⁎ |
| At-risk ascending aortic aneurysm | 17 (43%) | 8 (22%) | |
| Descending aortic aneurysm | 6 (15%) | 4 (11%) | NS ⁎ |
| At-risk descending aortic aneurysm | 8 (20%) | 7 (19%) |
⁎ Comparison based on combination of aortic complication and at-risk abnormality.
No patient met criteria for recoarctation. There were 15 patients with at-risk recoarctation ( Figure 2 ) including 14% of those undergoing Sc-CMR. Bivariate analysis showed significant predictors that included heart failure symptoms (odds ratio [OR] 7.4, 95% confidence interval [CI] 1.1 to 49.0, p = 0.039) and female gender (OR 3.3, 95% CI 1.0 to 10.9, p = 0.049).
Ascending aorta aneurysm was found in 1 patient undergoing Sc-CMR. At-risk ascending aorta enlargement was found in 25 patients, including 22% of Sc-CMR studies. Significant predictors included echocardiographic identification of aortic root enlargement (OR 22.9, 95% CI 2.6 to 198.9, p = 0.049) and time from repair (OR 1.1, 95% CI 1.0 to 1.1, p = 0.034).
Descending aorta aneurysm ( Figure 3 ) was found in 10 patients (4 Sc-CMR vs 6 Sx-CMR), and at-risk enlargement was found in 15 patients (7 Sc-CMR vs 8 Sx-CMR). Blood pressure, cardiovascular symptoms, and echocardiogram were not predictive. However, patients with bicuspid aortic valve were significantly more likely to have descending aorta enlargement (OR 4.3, 95% CI 1.5 to 12.3, p = 0.006).
Overall, aortic complications or at-risk abnormalities were found in 45 patients (59%) including 53% of those undergoing Sc-CMR. There were 18 patients with multiple abnormal aortic findings ( Figure 4 ). There were no significant differences in prevalence of aortic complications and abnormalities in patients undergoing Sc-CMR versus Sx-CMR ( Table 2 ).




