Increased plasma levels of neuron-specific enolase (NSE) are related to damage of neurons and neuroendocrine cells. We aimed to investigate elevation of NSE after elective percutaneous coronary intervention (PCI) on the prediction of silent cerebral infarct (SCI). Study population consisted of 2 groups of patients. Group 1 included 92 consecutive patients with normal coronary angiograms, whereas group 2 consisted of 89 patients who underwent elective coronary stenting. NSE levels were studied before and 12 hours after the procedure. Elevation of >0.12 μg/L was considered as SCI. Forty-seven of 181 study patients (26%) had SCI after the procedure. NSE elevation was significantly more prevalent in patients with PCI than that of controls. Elevation of NSE was observed in 42% of patients who underwent elective PCI (n = 37) and 11% of the normal coronary artery group (n = 10) (p <0.001). The incidence of SCI was higher in active smokers and patients who had history of myocardial infarction (MI) (55% vs 10%, p <0.001 for active smokers and 40% vs 8%, p <0.001 for history of MI, respectively). Multivariate analysis demonstrated history of smoking (odds ratio [OR] 9.9; 95% confidence interval [CI] 3.7 to 26.9; p <0.001) and previous MI (OR 4.4; 95% CI 1.7 to 11.4; p = 0.01) as independent predictors of SCI. For patients who underwent elective PCI, NSE levels after procedure increases. Invasive coronary procedures have risk of SCIs, even in patients with normal coronary arteries. In conclusion, increased diagnosis of SCIs might improve understanding of their relation with invasive cardiac procedures, facilitate to prevent occurrence of silent microemboli and decrease the risk of adverse neurologic events.
Neuron-specific enolase (NSE) is an intracytoplasmic glycolytic enzyme found in neurons and neuroendocrine tissues. Elevation of NSE in the absence of any clinically apparent stroke or transient ischemic attack, so-called silent cerebral infarcts (SCIs), may be associated with neurologic deficits, cognitive decline, psychiatric disorders (i.e., depression), clinically apparent stroke, and even increased mortality. Although rare, PCIs are known to cause cerebral embolic events. However, their role in the genesis of SCIs is not well documented. In the present study, we aim to evaluate the incidence of SCIs, defined as an elevation of NSE after diagnostic coronary angiography and elective coronary stenting.
Methods
Study population consisted of 2 groups of patients. Group 1 included 92 consecutive patients with normal coronary angiograms (no >50% diameter stenosis in any of the major coronary artery or their branches); group 2 consisted of 89 patients who underwent elective coronary stenting. Exclusion criteria were (1) baseline NSE elevation, (2) acute coronary syndromes or cardiac surgery within 4 weeks, (3) left ventricular dysfunction (left ventricular ejection fraction <50%), (4) planned use of glycoprotein IIb/IIIa receptor inhibitors, (5) patients with recent cerebrovascular accident, intracranial hemorrhage, and head trauma, (6) central nervous system tumor, (7) degenerative central nervous system disorders, (8) neuroendocrine tumors, and (9) symptomatic peripheral vascular disease. All patients gave written informed consent, and local ethics committee approved the study protocol.
All patients (n = 181, PCI group, n = 89, normal coronary angiography group, n = 92) underwent coronary angiography according to Judkins technique through femoral or radial approach. Patients with PCI received 100 to 300 mg of aspirin and oral loading dose of 300 mg clopidogrel, administered 12 to 24 hours before coronary stenting. Unfractionated heparin was given at the beginning of the procedure to obtain activated clotted time levels between 250 and 300 seconds for procedures. Patients with critical stenosis (≥70% stenosis on any of the major coronary arteries or their branches) underwent PCI in the same session. Coronary stenting procedure was performed using low osmolar, nonionic contrast agents (iopromide) with standard percutaneous techniques by experienced operators. The inflation and stent protocol were left to the discretion of the operator.
Measurements of NSEs were obtained at baseline and 12 hours after the intervention by h-NSE kits (Diametra, Foligno, Italy) with direct immunoenzymatic colorimetric method on immunologic automated analyzer. Intra-assay and interassay coefficient variabilities were ≤4.4% and ≤11.2%. Laboratory upper limits of normal were 0.12 μg/L for NSE according to manufacturers’ instruction. SCI was defined as an NSE level of >0.12 μg/L after the intervention. All patients were followed up for the occurrence of inhospital clinical events comprising stroke, death, and acute myocardial infarction (MI). For each patient, risk factors including hypertension, diabetes mellitus, hypercholesterolemia, smoking status, and history of ischemic stroke were obtained.
All analyses were performed using an SPSS software package (version 16.0 for Windows, SPSS Inc., Chicago, Illinois). Data are expressed as numbers and percentages for discrete variables and as means ± SD for continuous variables. The chi-square analysis was used to assess the significance of differences between categorical variables. Continuous variables were compared by Student’s t test or Mann–Whitney U test. In univariate analysis, age, gender, body mass index, waist circumference, present cigarette smoking, presentation of hypertension, diabetes, history of MI, history of Coronary artery bypass grafting surgery, the plasma levels of total cholesterol, low- and high-density lipoprotein and triglyceride, use of statins, beta blockers, calcium channel blockers, nitrates, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers were assessed. A multivariable logistic regression analysis was performed to assess the independent predictors of NSE elevation after the procedure. Results with a p value <0.05 were considered significant.
Results
Baseline clinical, angiographic, and procedural characteristics of study patients are seen in Table 1 . Stenting procedure was successful in all the cases with a residual diameter stenosis of <20% and thrombolysis in MI flow grade 3 after the intervention.
| Variable | Normal coronary angiography (n=92) | Elective percutaneous coronary intervention (n=89) | P value |
|---|---|---|---|
| Age, mean ± SD | 61±11 | 62±10 | 0.5 |
| Women | 58(63%) | 21(24%) | <0.001 |
| Body Mass Index (kg/m 2 ) | 27±5 | 27±5 | 0.6 |
| Waist circumference, (cm) | 117±8 | 102±13 | <0.001 |
| Hypertension ∗ | 61(66%) | 68(76%) | 0.1 |
| Diabetes Mellitus | 25(27%) | 36(40%) | 0.06 |
| Smoker | 6(7%) | 34(38%) | <0.001 |
| Hyperlipidemia † | 37(40%) | 56(63%) | 0.002 |
| Prior myocardial infarction | 0 (0%) | 29(33%) | <0.001 |
| Prior coronary bypass | 0 (0%) | 12 (14%) | <0.001 |
| Creatinine (mg/dl) | 0.8±0.2 | 1.1±1.0 | 0.02 |
| Total cholesterol (mg/dl) | 186±42 | 182±37 | 0.5 |
| Low-density lipoprotein (mg/dl) | 110±37 | 113±39 | 0.6 |
| High-density lipoprotein (mg/dl) | 46±15 | 45±16 | 0.6 |
| Triglyceride (mg/dl) | 163±33 | 142±63 | 0.6 |
| Baseline medication | |||
| Statin | 51(55%) | 67(75%) | 0.005 |
| Beta-blocker | 40(44%) | 53(60%) | 0.02 |
| Nitrate | 14(15%) | 54(61%) | <0.001 |
| Angiotensin-converting enzyme inhibitors/ Angiotensin receptor blockers | 50(53%) | 66(75%) | 0.003 |
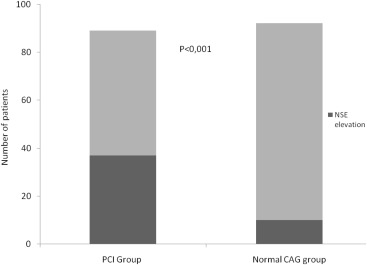
| Silent cerebral infarct (+), (Neuron specific enolase ≥0.12) | 10(11%) | 37(42%) | <0.001 |
∗ Blood pressure >140/90 mm Hg at 2 occasions or antihypertension treatment.
† Fasting low-density lipoprotein (LDL) level >160 mg/dL or patients on antihyperlidemia treatment.
Forty-seven of 181 study patients (26%) had SCI after the procedure. SCI was more prevalent in patients with PCI than that of those with normal coronary angiograms (42% vs 11%, p <0.001; Figure 1 ). When patients were divided into 2 groups according to SCI occurrence, those with SCI were more likely to have history of smoking and previous MI and less waist circumference ( Table 2 ).
| Variable | Silent cerebral infarct (+) (n=47) | Silent cerebral infarct (-) (n=134) | P value |
|---|---|---|---|
| Age, mean ± SD (years) | 59±10 | 62±11 | 0.2 |
| Women | 14(30%) | 65(49%) | 0.3 |
| Body Mass Index (kg/m 2 ) | 28±5 | 28±5 | 0.6 |
| Waist circumference, cm(Mean±SD) | 103±12 | 109±14 | 0.02 |
| Hypertension | 36 (77%) | 93 (69%) | 0.3 |
| Diabetes Mellitus | 12 (26%) | 49 (37%) | 0.2 |
| Smoker | 26(55%) | 14(10%) | <0.001 |
| Hyperlipidemia | 29(62%) | 64(48%) | 0.1 |
| Prior myocardial infarction | 19 (40%) | 10(8%) | <0.001 |
| Prior coronary bypass | 5 (11%) | 7 (5%) | 0.3 |
| Coronary artery stented: | |||
| Left anterior descending | 21 (57%) | 25 (48%) | 0.4 |
| Left circumflex | 15 (41%) | 25 (48%) | 0.5 |
| Right | 16 (43%) | 19 (37%) | 0.5 |
| Saphenous graft | 1 (9%) | 2 (20%) | 0.6 |
| Procedural characteristics: | |||
| Multivessel disease | 20(54%) | 32(62%) | 0.3 |
| Stent number (Mean±SD) | 1.9±1.2 | 1.7±0.9 | 0.4 |
| Drug eluting stent (Mean±SD) | 0.7±0.9 | 0.7±0.8 | 0.7 |
| Bare metal stent (Mean±SD) | 1.1±1.4 | 1±1.1 | 0.5 |
| Stent length (Mean±SD) | 32±23 | 26±16 | 0.1 |
| Stent size (Mean±SD) | 2.9±0.4 | 3±0.4 | 0.4 |
| Inflation pressure (Mean±SD) | 15±3 | 14±2 | 0.2 |
| Stenosis (Mean±SD) | 85±16 | 83±13 | 0.4 |
| Left ventricle ejection fraction (%) | 56±4 | 58±5 | 0.7 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


