Anemic patients remain at increased risk of ischemic and bleeding events. Whether the effects of hemoglobin levels on thrombotic and bleeding risk are independent of platelet reactivity on clopidogrel, however, remains unknown. Patients from the Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents study were categorized by the presence of anemia at baseline, defined according the World Health Organization criteria. Platelet reactivity was measured with VerifyNow assay; high platelet reactivity (HPR) on clopidogrel was defined as platelet reactive units value >208. Of 8,413 patients included in the study cohort, 1,816 (21.6%) had anemia. HPR was more prevalent in patients with anemia (58.3% vs 38.4%; p <0.001), an association that persisted after multivariate adjustment (adjusted odds ratio: 2.04; 95% confidence interval [CI]: 1.82 to 2.29; p <0.0001). Patients with anemia had higher 2-year rates of major adverse cardiac events (9.5% vs 5.6%; p <0.0001), major bleeding (11.8% vs 7.7%; p <0.0001), and all-cause mortality (4.0% vs 1.4%; p <0.0001); however, after adjustment for baseline clinical confounders, including HPR, anemia was no longer significantly associated with major adverse cardiac events but was still independently associated with all-cause mortality (adjusted HR 1.61, 95% CI 1.23 to 2.12; p <0.0001) and major bleeding (adjusted HR 1.42, 95% CI 1.20 to 1.68; p <0.0001). The effect of HPR on clinical outcomes was uniform according to anemia status, without evidence of interaction. In conclusion, anemia independently correlated with HPR. After percutaneous coronary intervention with drug-eluting stents, anemia at baseline was significantly associated with higher 2-year hemorrhagic and mortality risk; conversely, its association with ischemic risk was attenuated after multivariate adjustment, including HPR.
With more than 1.5 billion people affected, anemia is one of the most common clinical conditions worldwide. Moreover, anemia is a clinical co-morbidity frequently encountered in patients with coronary artery disease undergoing percutaneous coronary intervention (PCI). In patients undergoing PCI, especially those presenting with an acute coronary syndromes, baseline hemoglobin levels have been observed to be strongly related to adverse prognosis with increased risk for ischemic and bleeding complications. Moreover, patients with anemia undergoing revascularization are often frail and subject to significant undertreatment compared with patients without anemia. Atherothrombotic events are caused by atherosclerotic plaque rupture followed by thrombogenic pathways induction; a process in which, platelet activation plays a key role. Available evidence suggests that hemoglobin and erythropoietin levels modulate the propensity for intravascular thrombosis ; however, whether the effect of anemia on ischemia and bleeding is independent from the degree of pharmacological platelet inhibition and other concurrent clinical co-morbidities is currently unclear. Given the risks and benefits of dual antiplatelet therapy (DAPT) after drug-eluting stent (DES) implantation, an accurate characterization of the hemorrhagic and thrombotic risk of anemic patients undergoing PCI is crucial to optimize mid- and long-term outcomes. Therefore, in the present analysis from the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents (ADAPT-DES) study, we sought to characterize the impact of anemia at baseline on residual platelet reactivity on clopidogrel and its effect on thrombotic and hemorrhagic risk.
Methods
The ADAPT-DES study was a prospective, multicenter registry specifically designed to determine the association between platelet reactivity and stent thrombosis (ST) after DES implantation. The design and major outcomes of ADAPT-DES have been previously described. In brief, a total of 8,582 all-comers patients were prospectively enrolled at 11 sites in US and European hospitals. All patients who were successfully treated with 1 or more DES and who were adequately loaded with aspirin and clopidogrel were eligible for enrollment, regardless of clinical presentation or lesion complexity. The only major exclusion criteria were the occurrence of a major complication during the procedure or before platelet function testing, or if bypass surgery was planned after PCI. In addition, patients with hemoglobin <10 mg/dl and platelet count <100,000/mm 3 were excluded from the study cohort. Platelet reactivity on aspirin and clopidogrel was assessed after an adequate loading period to ensure full antiplatelet effect using the VerifyNow Aspirin, P2Y12, and IIb/IIIa assays (Accumetrics, San Diego, California). After PCI, patients were treated with aspirin indefinitely, and clopidogrel was recommended for at least 1 year. All other treatments were as per standard of care. Clinical follow-up was scheduled at 30 days, 1 year, and 2 years. An independent clinical events committee blinded to platelet function testing results adjudicated all events using original source documents. The institutional review board at each participating center approved the study, and all eligible patients signed written informed consent before enrolment.
The objectives of the present study were to: (1) characterize the unadjusted and adjusted association between baseline anemia and high platelet reactivity (HPR); (2) evaluate the crude and independent effect of anemia on major adverse cardiac events (MACE) and major bleeding; and (3) investigate the impact of HPR in patients with and without anemia on clinical outcomes. Anemia was defined as a hemoglobin concentration ≤12.0 mg/dl for women and ≤13.0 mg/dl for men, according to the World Health Organization definition. HPR was defined as P2Y12 reaction unit (PRU) value of >208. The coprimary end points of interest were MACE and major bleeding. MACE was defined as the composite of cardiac death, myocardial infarction (MI), or definite or probable ST. End point definitions used in the ADAPT-DES study have been previously reported.
Descriptive statistics are presented as mean ± SD and were compared with the Student t test; categorical variables are reported as percentages and were tested with the chi-square test. Adjusted odds ratio (OR) to evaluate association between anemia and HPR were obtained with logistic regression modeling adjusting for the following confounders: age, gender, body mass index, ethnicity, a history of congestive heart failure, current smoker, peripheral arterial disease, diabetes mellitus, creatinine clearance, hyperlipidemia, hypertension, previous MI, previous coronary artery bypass surgery, previous PCI, ST-segment elevation MI, non–ST-segment elevation MI, prehospital admission proton pump inhibitor use, prehospital admission aspirin use, prehospital admission thienopyridine use, intraprocedural bivalirudin use, prehospital admission statin use, prehospital admission warfarin use, white blood cell count, platelet count, number of stents, number of vessels treated, and total lesion length. Crude and adjusted analyses evaluating the association between anemia and HPR were performed using both PRU thresholds of >208 and ≥230. Crude event rates at 2 years for clinical outcomes were estimated using the Kaplan–Meier method in those with and without anemia and after substratifying all participants by both anemia status and HPR (PRU >208). Event rates across groups were compared with the log-rank test. Cox proportional hazard regression modeling stratified by propensity score quintile for anemia was performed to estimate the adjusted hazard ratios (HRs) associated with anemia status and 2-year adverse events. With a second Cox regression model stratified by propensity score quintile for HPR, the adjusted HR associated with HPR and 2-year adverse events in patients with versus without anemia were estimated. Formal interaction testing was performed to investigate a potential differential effect of HPR on clinical outcomes according to anemia status. Statistical analyses were performed with SAS version 9.4 (SAS Institute, North Carolina).
Results
Of 8,413 patients enrolled in the ADAPT-DES study for which baseline hemoglobin or hematocrit was available, 1,816 (21.6%) had anemia. Baseline and procedural characteristics in patients with and without anemia are presented in Tables 1 and 2 . Within the anemia group, mean hemoglobin (g/dl) was 12.1 ± 1.0 and mean hematocrit (%) was 35.5 ± 2.9, reflecting a mildly anemic population. Patients with anemia were older, were more commonly affected by multiple cardiac and noncardiac co-morbidities, had lower ejection fraction, and showed features of more advanced coronary artery disease.
| Variable | Anemia | P Value | |
|---|---|---|---|
| Yes (n=1816) | No (n=6597) | ||
| Age (years) | 66.7±10.7 | 62.8±10.7 | <0.0001 |
| Men | 1360 (74.9%) | 4902 (74.3%) | 0.61 |
| Caucasian | 1480 (81.5%) | 5981 (90.7%) | <0.0001 |
| Non-Caucasian | 336 (18.5%) | 616 (9.3%) | <0.0001 |
| Body mass index (m/kg 2 ) | 29.2±6.0 | 29.5±5.6 | 0.08 |
| Diabetes mellitus | 823 (45.3%) | 1900 (28.8%) | <0.0001 |
| Insulin-dependent | 342 (18.8%) | 635 (9.6%) | <0.0001 |
| Peripheral artery disease | 272 (15.0%) | 588 (8.9%) | <0.0001 |
| Congestive heart failure | 247 (13.6%) | 440 (6.7%) | <0.0001 |
| Previous myocardial infarction | 547 (30.1%) | 1575 (23.9%) | <0.0001 |
| Previous coronary artery bypass grafting | 467 (25.7%) | 969 (14.7%) | <0.0001 |
| Previous percutaneous coronary intervention | 923 (50.8%) | 2686 (40.7%) | <0.0001 |
| Arterial hypertension | 1557 (85.7%) | 5148 (78.0%) | <0.0001 |
| Chronic kidney disease ∗ | 515 (28.4%) | 851 (12.9%) | <0.0001 |
| Hyperlipidemia | 1458 (80.3%) | 4816 (73.0%) | <0.0001 |
| Cigarette smoker | 984 (54.2%) | 3755 (56.9%) | 0.03 |
| Current | 275 (15.1%) | 1635 (24.8%) | <0.0001 |
| Prior | 709 (39.0%) | 2120 (32.1%) | <0.0001 |
| Clinical presentation | |||
| Asymptomatic coronary artery disease | 294 (16.2%) | 758 (11.5%) | <0.0001 |
| Stable angina pectoris | 474 (26.1%) | 1991 (30.2%) | <0.0001 |
| Unstable angina pectoris | 567 (31.2%) | 1744 (26.4%) | <0.0001 |
| Non–ST-elevation myocardial infarction | 327 (18.0%) | 893 (13.5%) | <0.0001 |
| ST-elevation myocardial infarction | 106 (5.8%) | 678 (10.3%) | <0.0001 |
| NYHA class II–IV | 1328 (73.1%) | 4342 (65.8%) | <0.0001 |
| Ejection fraction (%) | 52.6 ± 12.5 | 55.5 ± 12.3 | <0.0001 |
| Laboratory data | |||
| Hemoglobin (g/dL) | 12.1 ± 1.0 | 14.5 ± 1.2 | – |
| Hematocrit (%) | 35.5±2.9 | 42.6±3.2 | – |
| White blood cells (1000/mL) | 7.50±4.37 | 8.07±2.78 | <0.0001 |
| Platelet count (1000/mm 3 ) | 222.8±69.1 | 227.7±61.0 | <0.0001 |
∗ Creatinine clearance <60 ml/min calculated by Cockcroft–Gault formula.
| Variable | Anemia | P Value | |
|---|---|---|---|
| Yes (n=1816) | No (n=6597) | ||
| Vascular access site | |||
| Femoral | 1698 (93.5%) | 6323 (95.8%) | <0.0001 |
| Radial | 8 (0.4%) | 10 (0.2%) | 0.04 |
| Number of coronary arteries treated | |||
| 1 | 624 (34.4%) | 2592 (39.3%) | 0.0001 |
| 2 | 598 (32.9%) | 2173 (32.9%) | 0.99 |
| 3 | 594 (32.7%) | 1832 (27.8%) | <0.0001 |
| Left main disease | 81 (4.5%) | 172 (2.6%) | <0.0001 |
| Number of lesions per patient | 1.5±0.8 | 1.5±0.8 | 0.58 |
| Total lesion length (mm) | 26.7±19.1 | 27.2±20.3 | 0.29 |
| Number of stents per patient | 1.67±0.96 | 1.71±1.02 | 0.22 |
| Total stent length (mm) | 31.8±21.4 | 32.7±22.7 | 0.12 |
| Thrombus | 168 (9.3%) | 1074 (16.3%) | <0.0001 |
| Calcified lesion | 564 (31.1%) | 2041 (30.9%) | 0.92 |
| Bifurcation lesion | 265 (14.6%) | 1034 (15.7%) | 0.25 |
| Chronic total occlusion | 79 (4.4%) | 319 (4.8%) | 0.38 |
| Coronary location of narrowing | |||
| Left anterior descending | 806 (44.4%) | 3072 (46.6%) | 0.09 |
| Right | 637 (35.1%) | 2484 (37.7%) | 0.04 |
| Left circumflex | 606 (33.4%) | 2003 (30.4%) | 0.01 |
| Left main | 89 (4.9%) | 223 (3.4%) | 0.003 |
| Intravascular ultrasound use | 645 (35.5%) | 2634 (39.9%) | 0.0006 |
Results from the platelet function testing are reported in Table 3 . Patients with anemia had higher levels of PRU and prevalence of HPR (58.3% vs 38.4%; p <0.001). After multivariate adjustment for baseline confounders, anemia was significantly associated with HPR (adjusted OR 2.04, 95% CI 1.82 to 2.29; p <0.0001). Results were consisted using a threshold of PRU ≥230 (data not shown). After stratification by moderate or severe chronic kidney disease (CKD; creatinine clearance <45 ml/min) status, patients with anemia and CKD had the highest PRU value (229.7 ± 104.7), whereas those without anemia and CKD had the lowest (176.0 ± 91.6; p trend <0.0001). By multivariate logistic regression analysis, the effect of anemia on HPR was consistent between patients with (adjusted OR 1.80, 95% CI 1.43 to 2.28) versus without CKD (adjusted OR 2.03, 95% CI 1.79 to 2.32; p interaction = 0.26).
| Variable | Anemia | P Value | |
|---|---|---|---|
| Yes (n=1816) | No (n=6597) | ||
| VerifyNow P2Y12 reaction units at baseline | 348.1±52.2 | 299.0±55.0 | <0.0001 |
| VerifyNow P2Y12 reduction, % | 35.7±27.7 | 41.2±28.3 | <0.0001 |
| VerifyNow P2Y12 reaction units on clopidogrel | 225.8±102.6 | 177.5±92.4 | <0.0001 |
| ≥230 | 929 (51.2%) | 2017 (30.6%) | <0.0001 |
| >208 | 1059 (58.3%) | 2534 (38.4%) | <0.0001 |
| Aspirin reaction units | 430.8±59.3 | 416.0±53.7 | <0.0001 |
| ≥550 | 138 (7.6%) | 333 (5.1%) | <0.0001 |
Medications used over the study period in patients with and without anemia are reported in Supplementary Table 1 . Patients with anemia were less commonly on aspirin at both 1 and 2 years. Conversely, although there was no difference in DAPT use at 1 year, patients with anemia were more commonly on DAPT at 2 years. Patients with anemia were also more commonly on warfarin and proton pump inhibitors through the study period.
Unadjusted and adjusted clinical outcomes at 2 years are presented in Table 4 . Patients with anemia had higher crude rates of MACE, major bleeding, and all-cause mortality. There were no differences in in-hospital bleeding risk between patients with versus without anemia ( Supplementary Table 2 ). Conversely, patients with anemia had higher risk of out-of-hospital bleeding (10.8% vs 6.1%; p <0.0001). The most common out-of-hospital bleeding site in both groups was gastrointestinal in origin, which was ≈twofold more frequent in patients with anemia (4.2% vs 2.0%; p <0.0001). Moreover, compared with patients without anemia, patients with anemia had a ≈2.5-fold increased risk of receiving a blood transfusion ( Supplementary Table 2 ).
| Variable | Anemia | Unadjusted Hazard Ratio [95% Confidence Interval] | P Value ∗ | Adjusted Hazard Ratio [95% Confidence Interval] | P Value † | |
|---|---|---|---|---|---|---|
| Yes (n=1816) | No (n=6597) | |||||
| All-cause mortality | 7.2% (125) | 2.9% (179) | 2.59 [2.06-3.26] | <0.0001 | 1.61 [1.23-2.12] | 0.0006 |
| Major bleeding ‡ | 11.8% (214) | 7.7% (507) | 1.53 [1.32-1.78] | <0.0001 | 1.42 [1.20-1.68] | <0.0001 |
| Major adverse cardiac events § | 9.5% (163) | 5.6% (354) | 1.70 [1.42-2.05] | <0.0001 | 1.22 [0.98-1.51] | 0.07 |
| Cardiac mortality | 3.9% (66) | 1.6% (99) | 2.47 [1.81-3.38] | <0.0001 | 1.38 [0.96-1.98] | 0.09 |
| Stent thrombosis ¶ | 1.4% (25) | 1.0% (66) | 1.39 [0.88-2.21] | 0.16 | 1.19 [0.70-2.02] | 0.52 |
| Myocardial infarction | 6.8% (115) | 4.2% (268) | 1.59 [1.27-1.97] | <0.0001 | 1.22 [0.95-1.58] | 0.11 |
‡ Reported as proportions with % (n) and relative risk.
§ Defined as the composite of cardiac mortality, definite or probable stent thrombosis, and myocardial infarction.
After multivariate adjustment ( Table 4 ), anemia was significantly associated with a higher risk of major bleeding (adjusted HR 1.42, 95% CI 1.20 to 1.68; p <0.0001) and all-cause mortality (adjusted HR 1.61, 95% CI 1.23 to 2.12; p = 0.0006); conversely, anemia was no longer associated with a higher risk of MACE. Anemia also had no effect on the risk of definite or probable ST or MI after multivariate adjustment. Independent correlates of MACE, bleeding, and mortality are reported in Supplementary Table 3 .
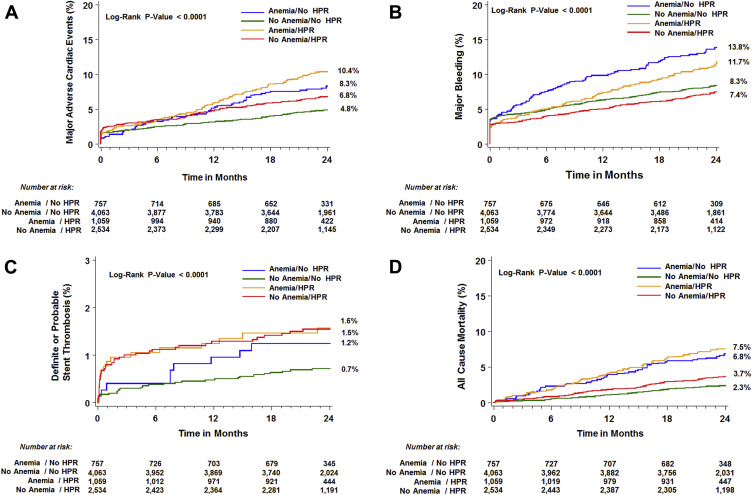
Two-year outcomes according to the presence of HPR in patients with and without anemia are reported in Figure 1 and Table 5 . Rates of MACE were highest in patients with anemia and HPR and lowest in those without anemia and without HPR ( Figure 1 ; trend p <0.0001). Conversely, rates of major bleeding were highest in patients with anemia and no HPR, whereas lowest in patients without anemia with HPR ( Figure 1 ; trend p <0.0001). Kaplan–Meier curves for definite or probable ST and all-cause mortality according to anemia and HPR status are shown in Figure 1 . By Cox regression modelling, the effect of HPR on clinical outcomes was uniform between patients with and without anemia ( Table 5 ), without evidence of interaction.