Although intraprocedural coronary computed tomographic angiography (CCTA) allows for scanning during intervention without relocation of the patient, studies have yet to report on its use during chronic total occlusion (CTO) intervention. Therefore, we investigated the role of CCTA during CTO intervention, particularly whether CCTA could be used to evaluate the location of guidewires. A total of 61 patients scheduled for elective CTO intervention were consecutively enrolled and underwent CCTA and on-site analyses during intervention. Transverse axial and the curved multiplanar images in a 360-degree view were interactively used together to identify the location of guidewires, along with the adjustment of window condition. Intracoronary contrast injection was used for specific cases requiring enhancement of the distal part of the CTO. Most CCTAs were performed to confirm the location of a single guidewire; CCTA was also performed to evaluate parallel (3 patients) or retrograde wires (5 patients). The initial identification rate for guidewire location was 56% with immediate transaxial images, but it significantly increased to 87% after interactive on-site uses of the curved multiplanar images (p <0.001). Cases in which guidewire location could be predicted with CCTA evaluation show a numerically higher success rate than those that could not (83% vs 63%) but not statistical significance (p = 0.174). The mean time for CCTA evaluation and mean radiation dose were 8.6 minutes and 2.9 mSv, respectively. No specific complications occurred after CCTA and CTO procedures. Intraprocedural CCTA for identifying the location of the guidewires is feasible and safe when used for various CTO procedural steps.
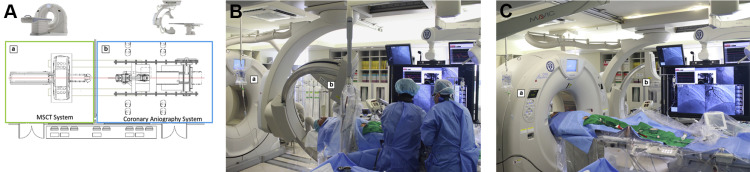
Guidewire crossing is the key to successful chronic total occlusion (CTO) recanalization. The use of intraprocedural imaging devices (e.g., intravascular ultrasound) is helpful, but CTO lesions can be fully evaluated only after guidewire and imaging catheter crossing through the CTO. Moreover, the tip of a guidewire cannot be evaluated under side mirroring systems equipped with current imaging devices. We built a coronary computed tomographic angiography (CCTA) system in the catheterization room (Yonsei University College of Medicine, Seoul, Korea) that allows for CCTA and coronary angiogram to be performed without taking patients off the table at any time during the intervention. Although preprocedural CCTA is helpful for CTO intervention, no data exist regarding the use of intraprocedural CCTA during CTO procedures. We investigated the role of intraprocedural CCTA during CTO intervention, with emphasis on whether CCTA could be used to evaluate the location and path of the CTO guidewires.
Methods
A prospective single-center design was used for this study. From January 2014 to December 2014, patients scheduled for CTO intervention who had no specific contraindications for intraprocedural CT scanning were consecutively enrolled. CTO was defined as obstruction of a native coronary artery with Thrombolysis In Myocardial Infarction flow grade 0 and an estimated duration ≥3 months based on the clinical history or previous coronary angiography results. All patients had typical chest pain or positive stress test results in various functional studies. According to Japan–Chronic Total Occlusion score, CTO lesions were classified as easy (score = 0), intermediate (score = 1), difficult (score = 2), and very difficult (score ≥3). The Institutional Review Board of the Yonsei University College of Medicine approved this study. Each enrolled patient received a detailed explanation of the study and provided written informed consent to participate.
We recently reported the first CTO case using the CCTA system. The CCTA system comprises a 640 multislice CT scanner applying double-slice technology (Aquilion ONE; Toshiba Medical Systems, Otawara, Japan) and a coronary angiography system and allows for scanning to be performed during intervention without moving the patient on the table ( Figure 1 ). The CT system has a wide detector width of 16 cm, which allows for a full cardiac CT data set to be acquired within a single heartbeat. In the present study, CT scan was performed using a cranial-to-caudal acquisition with prospective electrocardiogram gating using the following parameters: collimation and slice thickness, 0.5 mm; reconstruction increment, 0.5 mm; tube rotation time, 0.275 seconds; tube voltage, 100 kV(p); current, dose modulation; and reconstruction field of view, 180 mm. The data were reconstructed at 75% of the RR length. If motion artifacts were present, a different cardiac phase was selected. The following modulation was applied to the reconstruction: kernel, FC04; reconstruction algorithm, adaptive iterative dose reduction 3D. Sharp kernel with beam hardening collection was used to reduce metal artifacts. For intraprocedural CCTA scanning, the coronary angiogram system moved backward, and the CT system moved forward for scanning ( Figure 1 ). After scanning, the system operated in the reverse order, and on-site analyses of the CCTA images were performed.
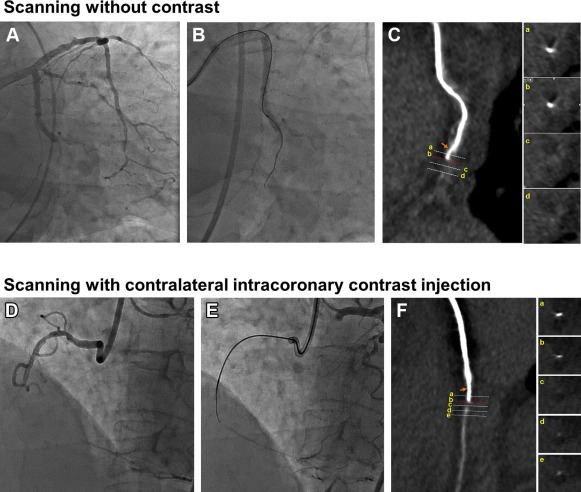
For specific cases that required enhancement of the distal part of the CTO, intracoronary contrast injection was used during CCTA ( Figure 2 ). The contrast medium (Visipaque 320 mg/ml; GE healthcare, Princeton) was diluted with normal saline solution to obtain optimal coronary enhancement (300 to –400 HU). The concentration of diluted contrast medium is 12.61 mgI/ml to produce 400 HU of luminal attenuation under the 100 kVp. The diluted contrast medium was delivered with a dual-head power injector (Medrad Stellant Injector; Medrad, Indianola, Pennsylvania) using the following protocols; injection rate of 5 ml/s and volume of contrast medium of 30 ml. The start buttons for the CT and the injector were pressed at the same time for CT scan. The CT scan started 2 seconds after the start button was pressed. The CT scan time was 1.176 seconds, and injection was continued for 6 seconds.
Before the CCTA, each patient’s vital signs were evaluated, and a beta-adrenergic blocker (esmolol, 1 mg/kg) was administered intravenously if the heart rate was ≥65 beats/min, and there were no contraindications to the use of beta-adrenergic blocking agents. The dose length product (mGy·cm) for CCTA was recorded. All radiation doses are presented as mSv, calculated as dose length product (DLP) × 0.014. The mean times for scanning and moving of CCTA system, as well as time for CCTA analyses, including data transfer and the total radiation doses were investigated.
Selected CCTA images were transferred to a workstation (Vitrea fX 6.4; Vital Images, Minnetonka, Minnesota), and then analysis for the identification of guidewire locations was performed. The double oblique and curved multiplanar reconstruction (C-MPR) images were obtained throughout the course of the coronary artery segments using a guidewire and were used for the examination of guidewire location in a 360-degree view with the transverse axial images ( Figure 3 ). In addition, the window width and level was adjusted to distinguish between the guidewire and other structures.
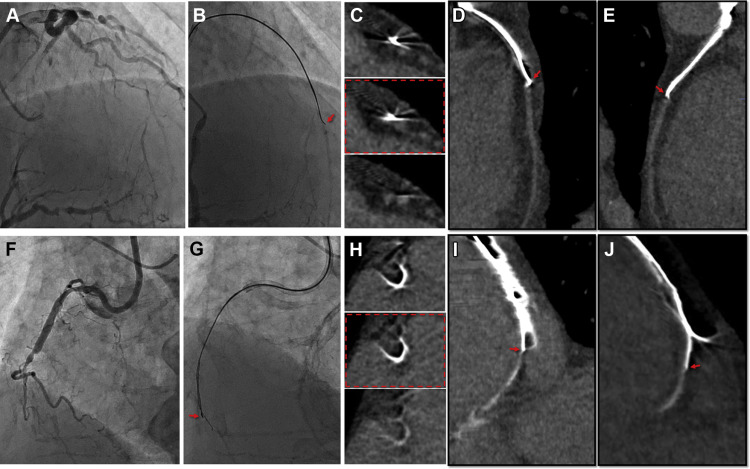
By on-site review of these various results, we discriminated guidewires from the vessel structures and investigated guidewire locations on longitudinal and cross-sectional sections. Based on the findings of CCTA, we predicted the possible locations of the guidewire tip divided into 3 different zones ( Figure 4 ): (1) intraplaque zone (suggestive of true lumen), case in which guidewires were located inside the vessel and clearly differentiated from vessel wall; (2) subintimal zone (suggestive of false lumen); cases in which the tip of the guidewire deviated to the lateral vessel wall and was not differentiated from the vessel wall; and (3) outside-vessel zone, cases in which the guidewire was completely out of the vessel wall.

Before coronary intervention, all patients received ≥75 mg aspirin, and a loading dose of 300 mg clopidogrel was administered at least 12 hours before the procedure. The choice of vascular access, CTO intervention techniques/devices, and the use of intravascular ultrasound were left to the discretion of the operators. Successful CTO intervention was defined as a final Thrombolysis In Myocardial Infarction flow grade of 3 and residual stenosis ≤30% by visual assessment after stent implantation.
Continuous variables are expressed as a mean ± SD, and categorical variables are presented as a number (%) and compared by the chi-square test or Fisher’s exact test. McNemar’s test was used for comparisons of changes in incidences. Agreement regarding guidewire location was evaluated using Fleiss’ kappa for multiple raters. In detail, 4 different observers including 2 interventionists and 2 specialized imaging cardiologists with level 3 clinical competence in cardiovascular CT imaging, assessed the location of guidewires. p Values <0.05 were considered statistically significant. Analyses were carried out using SPSS, version 20 (IBM Corporation, Chicago, Illinois) and R, version 3.21 (R Development Core Team, Vienna, Austria).
Results
From January 2014 to May 2015, a total of 61 patients who were scheduled for elective CTO intervention were consecutively enrolled and underwent CCTA and on-site analyses of CCTA images during intervention. The results for the patients’ baseline characteristics and the procedures performed are presented in Table 1 .
| Variable | (N=61) |
|---|---|
| Age (years) | 61.5±10.5 |
| Men | 54 (89%) |
| Body mass index (kg/m 2 ) | 25.7±3.2 |
| Hypertension | 43 (71%) |
| Diabetes mellitus | 24 (39%) |
| Prior myocardial infarction | 8 (13%) |
| Prior percutaneous coronary intervention | 22 (36%) |
| Coronary bypass surgery | 3 (5%) |
| Cerebrovascular accidents | 4 (7%) |
| Ejection fraction (%) | 57±13 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 80.0±15.1 |
| Chronic total occlusion lesion characteristics | |
| CTO coronary artery | |
| Left anterior descending | 26 (43%) |
| Left circumflex | 7 (11%) |
| Right | 28 (46%) |
| Stumpless occlusion | 11 (18%) |
| Prior failed lesion | 6 (10%) |
| Bridging collaterals | 23 (38%) |
| Stent occlusion | 5 (8%) |
| Pre-procedural computed tomographic evaluation | 21 (34%) |
| Japan–chronic total occlusion score | |
| 0 | 4 (6%) |
| 1 | 12 (20%) |
| 2 | 14 (23%) |
| ≥3 | 31 (51%) |
| Chronic total occlusion procedures | |
| Approach | |
| Anterograde | 47 (77%) |
| Retrograde | 14 (23%) |
| Vascular accesses | |
| Single femoral artery | 12 (20%) |
| Both femoral arteries | 38 (62%) |
| Femoral and radial arteries | 11 (18%) |
| Contralateral angiogram | 49 (80%) |
| Use of intravascular ultrasound | 37 (61%) |
| Successful intervention | 49 (80%) |
| Total number of stents (n) | 1.9±0.7 |
| Mean stent diameter (mm) | 3.03±0.28 |
| Total stented length (mm) | 54.9±21.3 |
| Total procedure time (min) | 101.3±60.2 |
| Total contrast volume used (mL) | 361.3±112.6 |
| Total radiation dose (mSV) | 110.5±77.9 |
| Quantitative angiographic analyses | |
| Reference vessel diameter (mm) | 2.84±0.58 |
| Chronic total occlusion length (mm) | 22.6±10.9 |
| Total lesion length (mm) | 42.1±20.5 |
| Post-procedural minimum lumen diameter (mm) | 2.63±0.49 |
A summary of the CCTA evaluation and those results is presented in Table 2 . Patients’ vital signs were stable before and after CCTA evaluations. Intraprocedural CCTA was performed for various CTO types and procedures ( Figure 5 ). In addition to showing the anterograde single wire, CCTA revealed the locations of retrograde and double wires used for parallel wire techniques.
| Variable | 61 patients |
|---|---|
| Systolic/ Diastolic blood pressure (mmHg) | 137.7±20.2 / 67.8±10.1 |
| Heart rate (beats per minute) | 63.9±12.1 |
| Total number of evaluation | 72 |
| Time for evaluation (minutes) | |
| Time for scanning and moving computed tomographic system | 8.6±2.1 |
| Time for coronary computed tomographic angiographic analyses including data transfer | 8.5±1.9 |
| Total time, including test, system-moving, and analyses | 17.2±2.9 |
| Radiation dose (mSv) | 2.9±1.5 |
| Reasons for coronary computed tomographic angiography | |
| Penetration of proximal chronic total occlusion cap by guidewire | 13 (18%) ∗ |
| Location of guidewire within chronic total occlusion segment | 48 (67%) ∗ |
| Entrance of guidewire into distal true lumen | 11 (15%) ∗ |
| Multiple coronary computed tomographic angiography during intervention | 10 (16%) |
| Evaluation according to the change of the approaches or wiring techniques | 4 |
| Evaluation after guidewire progress | 6 |
| Use of contrast | |
| Non-contrast scanning | 32 (52%) |
| Scanning with intracoronary contrast injection | 29 (48%) |
| Contralateral | 27 |
| Ipsilateral | 2 |
| Total diluted contrast volume used (mL) | 28.5±9.7 |
| Actual contrast volume used (mL) | 1.1±0.4 |
| Status of guidewire during coronary computed tomographic angiography | |
| Single wire | 64 (89%) ∗ |
| Parallel wires | 3 (4%) ∗ |
| Anterograde and retrograde wires | 5 (7%) ∗ |
| Identification of the location of guidewire tip and path | |
| Initial identification only by transverse axial images | 34 (56%) |
| Final identification by multiple modalities | 53 (87%) † |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


