The objective of this study was to investigate the prognostic significance of 12-lead electrocardiogram (ECG) patterns in a large multicenter cohort of patients with hypertrophic cardiomyopathy; 1,004 consecutive patients with hypertrophic cardiomyopathy and a recorded standard ECG (64% men, mean age 50 ± 16 years) were evaluated at 4 Italian centers. The study end points were sudden cardiac death (SCD) or surrogates, including appropriate implanted cardiac defibrillator discharge and resuscitated cardiac arrest and major cardiovascular events (including SCD or surrogates and death due to heart failure, cardioembolic stroke, or heart transplantation). Prevalence of baseline electrocardiographic characteristics was: normal ECG 4%, ST-segment depression 56%, pseudonecrosis waves 33%, “pseudo–ST-segment elevation myocardial infarction (STEMI)” pattern 17%, QRS duration ≥120 ms 17%, giant inverted T waves 6%, and low QRS voltages 3%. During a mean follow-up of 7.4 ± 6.8 years, 77 patients experienced SCD or surrogates and 154 patients experienced major cardiovascular events. Independent predictors of SCD or surrogates were unexplained syncope (hazard ratio [HR] 2.5, 95% confidence interval [CI] 1.4 to 4.5, p = 0.003), left ventricular ejection fraction <50% (HR 3.5, 95% CI 1.9 to 6.7, p = 0.0001), nonsustained ventricular tachycardia (HR 1.7, 95% CI 1.1 to 2.6, p = 0.027), pseudo-STEMI pattern (HR 2.3, 95% CI 1.4 to 3.8, p = 0.001), QRS duration ≥120 ms (HR 1.8, 95% CI 1.1 to 3.0, p = 0.033), and low QRS voltages (HR 2.3, 95% CI 1.01 to 5.1, p = 0.048). Independent predictors of major cardiovascular events were age (HR 1.02, 95% CI 1.01 to 1.03, p = 0.0001), LV ejection fraction <50% (HR 3.73, 95% CI 2.39 to 5.83, p = 0.0001), pseudo-STEMI pattern (HR 1.66, 95% CI 1.13 to 2.45, p = 0.010), QRS duration ≥120 ms (HR 1.69, 95% CI 1.16 to 2.47, p = 0.007), and prolonged QTc interval (HR 1.68, 95% CI 1.21 to 2.34, p = 0.002). In conclusion, a detailed qualitative and quantitative electrocardiographic analyses provide independent predictors of prognosis that could be integrated with the available score systems to improve the power of the current model.
Hypertrophic cardiomyopathy (HC) is the most common genetic cardiac disease and one of the main causes of sudden cardiac death (SCD) in the young. Different noninvasive clinical and instrumental markers, derived from observational cohort studies, have been related to worse prognosis. However, risk stratification is still largely unsatisfactory because of the low positive predictive value of these predictors. In recent years, the standard electrocardiogram (ECG) has been shown to provide useful prognostic indications in patients with HC. A normal ECG is generally associated with a mild phenotype and favorable outcome, whereas marked electrocardiographic abnormalities have been shown to be correlated with severe hypertrophy and the presence of myocardial fibrosis. However, this universally available, low-cost, and reproducible technique has rarely been tested in multivariable models for SCD and major cardiovascular events’ risk stratification. In the present study, we aimed to conduct an extensive qualitative and quantitative electrocardiographic analysis to assess the independent long-term predictive value of specific ECG patterns for prognosis in a large, multicenter HC cohort.
Methods
In this retrospective observational study, we analyzed 1,047 consecutive patients evaluated from 1981 to 2011 at 4 Italian centers (S. Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy [n = 452], Sapienza University of Rome, Rome, Italy [n = 296], Azienda Ospedaliera Careggi, Florence, Italy [n = 233], Monaldi Hospital, University of Naples, Naples, Italy [n = 66]) with unequivocal diagnosis of HC and a good-quality12-lead ECG obtained at first evaluation. HC was diagnosed by the presence of hypertrophied and nondilated left ventricle (wall thickness ≥15 mm in adults) at transthoracic echocardiogram, in the absence of other cardiac or systemic conditions that could produce a comparable magnitude of LV hypertrophy. Forty-three patients (4%) were excluded for the following reasons: (1) inadequate technical electrocardiographic quality (n = 14); (2) presence of paced ventricular rhythm (n = 15); and (3) percutaneous septal alcohol ablation or myectomy preceding first evaluation (n = 14). Therefore, 1,004 patients constituted the final study population.
The study end points were (1) SCD or surrogates, defined as unexpected collapse occurring <1 hour from the onset of symptoms in a patient who had previously experienced a relatively uneventful clinical course or unexpected unwitnessed death or aborted cardiac arrest or appropriate implantable cardioverter defibrillator (ICD) therapy (for ventricular tachycardia/fibrillation with a heart rate ≥200 beats/min). (2) Major cardiovascular events, defined as SCD or surrogates, death due to heart failure or cardioembolic stroke, or heart transplantation. Follow-up was obtained by scheduled or nonscheduled clinical evaluations. For patients who had not been evaluated clinically for >1 year, follow-up was obtained by telephone interview or by contacting the general practitioner.
The scalar 12-lead ECGs (standard calibration 10 mm/1 mV) at first evaluation were performed on commercially available instruments in the supine position during quiet respiration and recorded at a paper speed of 25 mm/s. The following parameters were measured: PR interval, QRS duration, QRS voltages, QT interval, and corrected QT interval (Bazett’s formula). Left bundle branch block (LBBB) or right bundle branch block, left anterior fascicular block, and nonspecific intraventricular conduction disturbance were classified according to international criteria. A number of electrocardiographic criteria proposed for the clinical identification of LV hypertrophy were used, including Cornell voltage score (SV3 + R aVL ≥20 mm in women and ≥28 mm in men), modified Sokolow-Lyon score (SV1 or SV2 + RV5 or RV6 ≥35 mm), the amplitude of R wave in aVL (≥11 mm), and the sum of RDI and SDIII (≥25 mm). LV hypertrophy was diagnosed in the presence of at least 1 criteria. Massive LV hypertrophy was defined by modified Sokolow-Lyon score ≥50 mm.
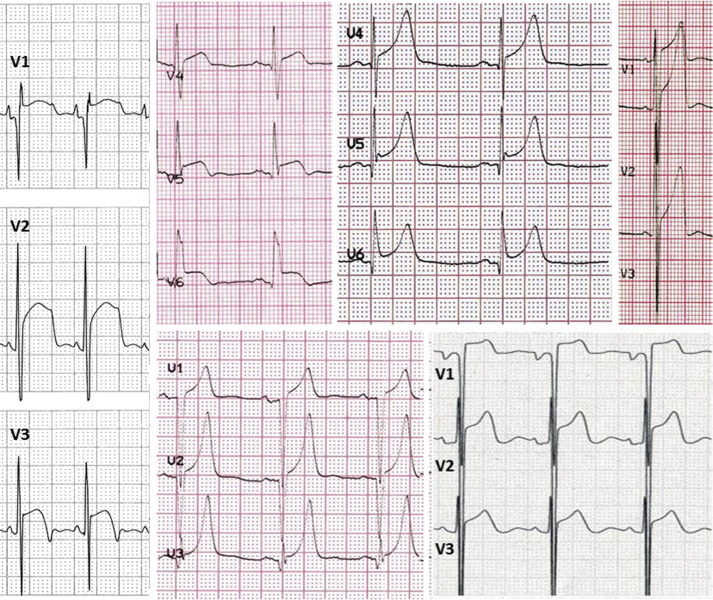
We defined specific ECG patterns. Pseudo-necrosis: presence of Q waves ≥1/3 of the ensuing R wave in depth and/or ≥0.04 seconds in duration in at least 2 contiguous leads except aVR and/or the lack of progressive R-wave voltage increase in the precordial leads. Low voltages: QRS complex amplitude <0.5 mV in all limb leads. Repolarization abnormalities: ST-segment depression/elevation ≥0.1 mV below or above the baseline at the J-point in at least 2 leads, except V1-V2-V3, where it was considered only when ≥0.2 mV. T waves were defined as (1) “inverted” when the negative T-wave amplitude was ≥0.1 mV, (2) “giant negative” when amplitude was ≥1.0 mV, and (3) “giant positive” T waves were defined as symmetrical positive T waves ≥1.0 mV. ST-segment elevation and giant positive T waves were considered only in the absence of LBBB. The presence of notching in terminal part of QRS was defined as “J wave.” A specific ECG pattern characterized by the presence of ST-segment elevation and/or giant positive T waves in at least 2 contiguous leads in the absence of LBBB was assessed and defined “pseudo–ST-segment elevation myocardial infarction (STEMI)” pattern for its resemblance of the ECG associated with the acute/subacute phase of myocardial infarction ( Figure 1 ). Other prespecified ECG patterns such as massive LV hypertrophy, massive LV hypertrophy associated with right atrial enlargement, and LV hypertrophy with abnormal R/S in V1 were assessed. All ECGs were independently analyzed using manual calipers, and patterns were adjudicated by 2 independent investigators (EB and CP—S.Orsola Malpighi Hospital Bologna) unaware of the clinical details of the patients. Discrepancies were resolved by a senior supervisor (CR).
Standard M-mode and 2-dimensional echocardiographic studies were performed by commercially available instruments to identify and qualify morphologic features of the left ventricle. The greatest wall thickness measured at any site in the LV wall was regarded as the maximal thickness, independently of body surface area and gender or age. LV outflow obstruction in basal conditions was defined as a peak outflow gradient of ≥30 mm Hg, as estimated by continuous-wave Doppler echocardiography.
Continuous data were expressed as mean value ± SD. Data were compared by the chi-square analysis for proportions and Student’s t test for continuous variables. Survival rates were obtained using Kaplan-Meier method of estimation. Univariate and multivariable Cox proportional hazard regression models were constructed to identify independent predictors of follow-up events. Each electrocardiographic variable was assessed by Cox proportional univariate survival analysis. Model building followed a backward stepwise approach with entry set at a significance level of 0.1. For each variable, the hazard ratio (HR) with a corresponding 95% confidence interval was reported. Harrell’s C index was used to assess model’s discrimination. A p value <0.05 was considered statistically significant. Statistical analyses were obtained using Stata/SE 13.1 (StataCorp IP, College Station, Texas).
Results
Clinical and echocardiographic characteristics and arrhythmic risk profile of the study population are listed in Table 1 . In our population, 29 patients had an ICD at first evaluation and 150 underwent ICD implantation during follow-up.
| Variable | |
|---|---|
| 1004 | |
| • Age ≥ 18 | 984 (98 %) |
| • Age 14-17 | 20 (2%) |
| Males | 640 (64%) |
| Age (years), mean ± SD | 50 ± 16 |
| Family history of HC | 441 (44%) |
| Family history of SCD | 196 (20%) |
| LV outflow tract obstruction (≥30 mmHg) | 335 (33%) |
| Unexplained syncope | 91 (9%) |
| NSVT on Holter monitoring | 281 (28%) |
| NYHA functional class III-IV | 71 (7%) |
| Maximal LV wall thicknes (mm) mean ± SD/n patients ∗ | 20 ± 5/997 |
| LV end-diastolic dimension, (mm) mean ± SD/n patients ∗ | 45 ± 7/955 |
| LV ejection fraction <50% | 51 (5%) |
| Dilated-hypokinetic evolution | 44 (4%) |
Electrocardiographic variables are listed in Table 2 . The pseudo-STEMI pattern was present in 171 patients (17%) including 97 patients with ST-segment elevation, 34 with giant positive T waves, and 40 with both. One hundred twenty-one patients (71%) showed this pattern in anterior leads, 27 patients (16%) in inferior leads, 15 patients (9%) in inferolateral leads, and 8 patients (5%) only in lateral leads. A short PR interval (≤120 ms) was present in 71 cases.
| Characteristics | Prevalence |
|---|---|
| Normal ECG | 38 (4%) |
| QRS duration ≥120msec | 172 (17%) |
| Prolonged corrected QT interval (≥440 msec) | 297 (30%) |
| Prolonged corrected QT interval (≥ 480 msec) | 65 (6%) |
| Left atrial enlargement | 448 (45%) |
| Right atrial enlargement | 85 (8%) |
| LV hypertrophy ∗ | 562 (56%) |
| Massive LV hypertrophy | 139 (14%) |
| Massive LV hypertrophy associated to right atrial enlargement | 11 (1%) |
| LV hypertrophy in presence of abnormal R/S in V1 | 23 (2%) |
| Low QRS voltages | 33 (3%) |
| Pseudo-necrosis Q waves | 335 (33%) |
| Repolarisation abnormalities | 778 (77%) |
| Negative T wave | 746 (74%) |
| Giant inverted T waves (≥10 mm) | 56 (6%) |
| Giant positive T waves (≥10 mm) | 74 (7%) |
| ST-segment depression | 557 (56%) |
| ST-segment elevation | 137 (14%) |
| “Pseudo-STEMI” pattern | 171 (17%) |
| ST segment elevation without giant positive T waves | 97 (10%) |
| Giant positive T waves without ST segment elevation | 34 (3%) |
| ST segment elevation and giant T waves | 40 (4%) |
| J wave | 34 (3%) |
| Abnormal R/S wave in V1 | 60 (6%) |
| Complete RBBB | 67 (7%) |
| Complete LBBB | 63 (6%) |
| Unspecified intraventricular block | 73 (7%) |
| Left anterior fascicular block | 133 (13%) |
After a mean follow-up of 7.4 ± 6.8 years, 77 patients experienced SCD or surrogates including 41 who actually died suddenly (4%), 24 with appropriate ICD discharge (2%), and 12 with aborted cardiac arrest (1%). A total of 154 patients (15%) experienced major cardiovascular events, including the 77 with SCD or surrogates, 60 (6%) with heart failure–related death (15 of which underwent heart transplantation), and 17 (2%) who died after cardioembolic stroke. The electrocardiographic findings associated with SCD or surrogates at univariate analysis were QRS ≥120 ms, pseudo-STEMI pattern, ST-segment elevation, and low QRS voltages ( Table 3 ). The electrocardiographic findings associated with major cardiovascular events at univariate analysis were QRS ≥120 ms, prolonged corrected QT interval, left and right atrial enlargement, and pseudo-STEMI pattern ( Table 3 ).
| Characteristics | SCD or surrogates Univariate Analysis | Major cardiovasculare events Univariate Analysis | ||
|---|---|---|---|---|
| H.R. (95% CI) | P value | H.R. (95% CI) | P value | |
| Age | 0.99 (0. 98 – 1.01) | 0.610 | 1.02 (1.01 – 1.03) | 0.0001 |
| Male | 0.83 (0.49 – 1.43) | 0.268 | 1.20 (0.87 – 1.66) | 0.286 |
| Family history of SCD | 1.66 (0.97 – 2.84) | 0.007 | 1.86 (1.33 – 2.61) | 0.0001 |
| Familiar HC | 1.92 (1.16 – 3.19) | 0.011 | 1.57 (1.14 – 2.16) | 0.006 |
| Unexplained syncope | 2.51 (1.34 – 4.73) | 0.004 | 1.48 (0.89 – 2.46) | 0.128 |
| NSVT 24 hour Holter monitoring | 1.81 (1.11 – 2.98) | 0.024 | 1.47 (1.07 – 2.65) | 0.018 |
| Maximal LV wall thickness | 1.38 (0.93 – 2.04) | 0.419 | 1.12 (0.85 – 1.46) | 0.420 |
| Maximal LV wall thickness ≥ 3 cm | 1.51 (0.74 – 3.06) | 0.274 | 1.40 (0.86 – 2.27) | 0.170 |
| LV end-diastolic dimension | 1.12 (0.72 – 1.75) | 0.017 | 1.71 (1.32 – 2.22) | 0.0001 |
| LV outflow tract obstruction | 1.05 (0.60 – 1.82) | 0.675 | 0.98 (0.67 – 1.42) | 0.905 |
| LV ejection fraction < 50% | 4.47 (2.44 – 8.18) | 0.0001 | 4.52 (2.95 – 6.94) | 0.0001 |
| Normal ECG | 0.35 (0.05 – 2.52) | 0.297 | 0.34 (0.08 – 1.39) | 0.135 |
| QRS duration ≥120msec | 2.09 (1.25 – 3.49) | 0.005 | 2.59 (1.84 – 3.66) | 0.0001 |
| Prolonged corrected QT interval (≥440 msec) | 1.56 (0.99 – 2.47) | 0.056 | 1.86 (1.35 – 2.56) | 0.0001 |
| Prolonged corrected QT interval (≥ 480 msec) | 1.23 (0.54 – 2.85) | 0.618 | 2.45 (1.58 – 3.80) | 0.0001 |
| Left atrial enlargement | 1.31 (0.79 – 2.16) | 0.288 | 1.53 (1.08 – 2.16) | 0.015 |
| Right atrial enlargement | 0.89 (0.36 – 2.21) | 0.803 | 1.85 (1.12 3.03) | 0.015 |
| LV hypertrophy ∗ | 0.90 (0.57 – 1.42) | 0.654 | 1.06 (0.76 1.46) | 0.733 |
| Massive LV hypertrophy | 0.97 (0.51 – 1.83) | 0.917 | 0.92 (0.58 – 1.46) | 0.724 |
| Massive LV hypertrophy associated to right atrial enlargement | 1.05 (0.15 – 7.46) | 0.956 | 1.68 (.53 – 5.28) | 0.375 |
| LV hypertrophy in presence of abnormal R/S in V1 | 0.66 (0.09 – 4.79) | 0.686 | 1.79 (0.73 – 4.37) | 0.201 |
| Low QRS voltages | 2.71 (1.24 – 5.91) | 0.012 | 1.80 (0.91 – 3.53) | 0.089 |
| Pseudo-necrosis Q waves | 1.35 (0.86 – 2.13) | 0.191 | 1.03 (0.74 – 1.43) | 0.858 |
| Repolarisation abnormalities | 1.09 (0.61 – 2.0) | 0.754 | 1.08 (0.71 – 1.63) | 0.715 |
| Negative T wave | 0.66 (0.36 – 1.19) | 0.170 | 0.85 (0.58 – 1.25) | 0.409 |
| Giant inverted T waves (≥10 mm) | 0.36 (0.09 – 1.46) | 0.152 | 0.44 (0.18 – 1.07) | 0.152 |
| Giant positive T waves (≥10 mm) | 0.94 (0.51 – 1.75) | 0.493 | 1.25 (0.72 – 1.73) | 0.407 |
| ST-segment depression | 1.07 (0.67 – 1.70) | 0.772 | 1.15 (0.83 – 1.61) | 0.389 |
| ST-segment elevation | 2.42 (1.50 – 3.89) | 0.004 | 1.45 (0.99 – 2.12) | 0.525 |
| “Pseudo-STEMI” pattern | 2.07 (1.26 – 3.38) | 0.0001 | 1.60 (1.07 – 2.35) | 0.020 |
| J wave | 1.93 (0.78 – 4.80) | 0.154 | 1.93 (0.78 – 4.80) | 0.154 |
| Abnormal R/S wave in V1 | 0.40 (0.09 – 0.64) | 0.207 | 1.03 (0.54 – 1.97) | 0.914 |
| Left anterior fascicular block | 0.87 (0.44 – 1.70) | 0.682 | 0.89 (0.56 – 1.42) | 0.646 |
At multivariable analysis ( Table 4 ), unexplained syncope (hazard ratio [HR] 2.47, 95% confidence interval [CI] 1.37 to 4.47, p = 0.003), nonsustained ventricular tachycardia (NSVT) during Holter monitoring (HR 1.68, 95% CI 1.06 to 2.65, p = 0.027), LV ejection fraction <50% (HR 3.55, 95% CI 1.89 to 6.66, p = 0.0001), pseudo-STEMI pattern (HR 2.28, 95% CI 1.38 to 3.77, p = 0.001), QRS duration ≥120 ms (HR 1.78, 95% CI 1.05 to 3.03, p = 0.033), and low QRS voltages (HR 2.26, 95% CI 1.01 to 5.07, p = 0.048) were independently associated with SCD or surrogates. Harrell’s C index was 0.68. Sensibility and specificity of QRS duration in predicting SCD were 26% and 84%, respectively; positive and negative predictive values were 12% and 93%, respectively. Sensibility and specificity of pseudo-STEMI pattern in predicting SCD were 30% and 84%, respectively; positive and negative predictive values were 13% and 93%, respectively. Sensibility and specificity of low QRS voltages in predicting SCD were 9% and 97%, respectively; positive and negative predictive values were 21% and 93% respectively.



