Pulmonary arterial hypertension (PAH) is a disorder in which pulmonary arterial remodeling and vasoconstriction progressively lead to right heart failure (HF), exercise intolerance, and high mortality. Beta-blockers have been shown to decrease mortality in left-sided HF, but their efficacy in isolated right HF associated with PAH is uncertain. Patients with PAH may have cardiac co-morbidities for which β-blocker therapy is indicated, and the relative risk benefit of this therapy remains to be proved. This is a prospective cohort study of 94 consecutive patients with PAH divided into 2 groups with and without β-blocker use at baseline. Rate of all-cause mortality, PAH-related hospitalization, change in 6-minute walk test, right ventricular structure and function measured by echocardiography, and hemodynamics measured by right heart catheterization were determined between subjects with and without β-blocker use. Beta-blocker use was common (28%) in this cohort. After a median follow-up of 20 months, changes in pulmonary hemodynamics and right ventricular size and function were similar between groups. There were no statistically significant differences in adverse events including PAH-related hospitalization or all-cause mortality (p = 0.19), presence of right HF by last visit (p = 0.75), or change in last 6-minute walk distance (p = 0.92). In conclusion, β-blocker use is not uncommon in a select group of patients with PAH and cardiac co-morbidities and did not appear to exert detrimental effects in clinical, functional, and hemodynamic outcomes. Further randomized data are needed to evaluate the potential benefits and risks of β-blocker use in patients with PAH.
In this prospective clinical study, we investigated whether the use of β-blockers was associated with an improvement in clinical, functional, and hemodynamic outcomes in patients with pulmonary arterial hypertension (PAH).
Methods
This is a prospective cohort study of 94 consecutive patients from the Ottawa Heart Institute Pulmonary Hypertension Clinic evaluated for the assessment/diagnosis of PAH. The investigation conformed with principles outlined in the Declaration of Helsinki and was approved by the research ethics board with informed consent obtained from all subjects. Patients were divided into 2 groups at baseline, those with and without current use of β-blocker therapy. Baseline was defined as the first available clinic visit data and outcome was defined as the last clinic visit data. All decisions on initiation, dosing, and discontinuation of β-blocker therapy were left to the discretion of the treating physician and documented. The overall aim of this study was to determine if there were differences in clinical event rates, changes in exercise capacity, right ventricular (RV) structure and function, and hemodynamics between patients with and without β-blocker treatment.
Patients were included in this study if they were diagnosed with World Health Organization group 1 PAH, defined as mean pulmonary arterial pressure >25 mm Hg at rest with mean pulmonary capillary wedge pressure ≤15 mm Hg and pulmonary vascular resistance ≥240 dyne/s/cm 5 . This included any patient whose PAH was judged to be secondary to familial or idiopathic cause or associated with congenital heart disease, connective tissue disease, human immunodeficiency virus, anorexigen use, or portal hypertension.
Patients were excluded if their PAH was caused primarily by (1) group 2 PAH or pulmonary venous hypertension from left-sided heart disease, (2) group 3 PAH from pulmonary disease including obstructive sleep apnea, chronic obstructive pulmonary disease, asthma, interstitial lung disease, or restrictive lung disease, or (3) group 4 PAH from chronic thrombotic and/or embolic disease.
Daily β-blocker dose (milligrams) was converted to a metoprolol-equivalent dose based on the following conversion factor: metoprolol 100 mg = atenolol 50 mg = bisoprolol 5 mg = propranolol 80 mg = acebutolol 200 mg = nadolol 80 mg.
Multiple clinical and hemodynamic outcomes were evaluated. These included all-cause mortality, hospital admission for PAH, or clinical right heart failure (HF). For this study, clinical right HF was defined as a patient with any of the following clinical signs: (1) increase in jugular venous pressure >4 cm above the sternal angle, (2) signs of peripheral edema (≥1+), (3) initiation or increase in a diuretic for HF, or (4) development of ascites. Hospital admission for PAH was defined as any admission in which the most responsible diagnosis was (1) decompensated right HF or (2) PAH requiring intensification of therapy. In addition, baseline and follow-up data were collected on New York Heart Association (NYHA) functional class, exercise capacity with the 6-minute walk test (6MWT), RV function and size assessed qualitatively by echocardiography. Hemodynamic data were obtained with serial right heart catheterizations when clinically indicated (e.g., diagnosis of PAH or worsening PAH or to evaluate response to treatment).
Demographic characteristics between the 2 groups (with and without β-blocker treatment) were analyzed using chi-square tests. Continuous variables that followed normal distribution were reported as mean ± SD, and parametric paired or unpaired t test was performed when appropriate. For ordinal/categorical variables or variables that did not follow a normal distribution, nonparametric Mann–Whitney U test was performed. A p value <0.05 was considered statistically significant. All statistical analyses were performed using STATA 9.2 (STATA Corp., College Station, Texas).
Results
Ninety-four patients were included in this analysis; 28% were taking β-blockers at their first clinic visit. Median follow-up time was 20 months and not different between groups. Demographics and baseline clinical characteristics were similar except for a larger percentage of patients with atrial fibrillation, hypertension, and coronary artery disease in the β-blocker group ( Table 1 ). Percentage of subjects showing clinical signs of right HF at baseline was not different between groups. As shown in Figure 1 , most subjects in the 2 groups had idiopathic PAH. However, the distribution of PAH subtype was different (p = 0.009), with more connective tissue disease in the group not on β-blockers and more portal hypertension in the β-blocker group.
| Characteristics | No Beta-Blocker | Beta-Blocker | p Value |
|---|---|---|---|
| (n = 68) | (n = 26) | ||
| Age (years), mean ± SD | 62.5 ± 17.6 | 69.3 ± 12.3 | 0.08 |
| Women | 52 (77%) | 15 (58%) | 0.07 |
| White race | 49 (72%) | 21 (81%) | 0.39 |
| Atrial fibrillation | 9 (13%) | 10 (39%) | 0.006 |
| Hypertension | 25 (37%) | 17 (65%) | 0.013 |
| Coronary artery disease | 4 (6%) | 10 (39%) | <0.001 |
| Type 2 diabetes mellitus | 13 (19%) | 9 (35%) | 0.11 |
| Aortic valve disease | 2 (3%) | 2 (8%) | 0.31 |
| Pulmonary disease | 27 (40%) | 7 (27%) | 0.25 |
| Right heart failure | 35 (52%) | 18 (69%) | 0.12 |
| Systolic blood pressure (mm Hg), mean ± SD | 125 ± 21 | 120 ± 25 | 0.42 |
| Diastolic blood pressure (mm Hg), mean ± SD | 72 ± 9 | 68 ± 11 | 0.08 |
| Heart rate (beats/min), mean ± SD | 83 ± 15 | 69 ± 11 | 0.0001 |
| Endothelin receptor antagonist | 15 (22%) | 1 (4%) | 0.036 |
| Sildenafil | 8 (12%) | 3 (12%) | 0.98 |
| Prostanoid | 2 (3%) | 0 (0%) | 0.38 |
| Calcium channel blocker | 24 (35%) | 4 (15%) | 0.059 |
| Furosemide | 25 (37%) | 10 (39%) | 0.88 |
| Spironolactone | 5 (7%) | 3 (12%) | 0.52 |
| Digoxin | 5 (7%) | 1 (4%) | 0.53 |
| Warfarin | 18 (27%) | 7 (27%) | 0.97 |
| Oxygen (%) | 11 (16%) | 4 (15%) | 0.93 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker (%) | 27 (40%) | 11 (42%) | 0.82 |

Table 2 presents β-blocker type, percentage, and median dose used in the β-blocker group. Reasons for using β-blockers included hypertension, atrial fibrillation, and/or coronary artery disease. Other reasons included esophageal varices and exercise-induced ventricular fibrillation and palpitation. Three subjects who did not receive β-blocker at baseline were started on a β-blocker during the study period. Reasons for starting β-blocker were hyperthyroidism and atrial fibrillation. Four subjects who received β-blocker therapy at baseline had stopped during the study period. Reasons for discontinuation of β-blocker therapy were atrial fibrillation with slow ventricular response and symptomatic lightheadedness.
| Type of β-blocker used | |
| Metoprolol | 54% |
| Atenolol | 8% |
| Bisoprolol | 27% |
| Propranolol | 4% |
| Acebutolol | 4% |
| Nadolol | 4% |
| Metoprolol-equivalent dose (mg), median (interquartile range) ⁎ | 100 (50–100) |
| Reason(s) for using β-blocker | |
| Hypertension | 33% |
| Atrial fibrillation | 13% |
| Coronary artery disease | 13% |
| Combinations of hypertension, atrial fibrillation, coronary artery disease | 25% |
| Esophageal varices | 8% |
| Exercise-induced ventricular fibrillation | 4% |
| Palpitation | 4% |
⁎ Metoprolol equivalent in milligrams per day (see Methods for conversion).
At baseline the proportion of patients on PAH-specific therapy (endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, or prostacyclin derivatives) tended to be larger in the group not on β-blockers ( Table 1 ). Three patients in the no–β-blocker cohort were on dual therapy compared to 0 in the β-blocker group (p = 0.27). By the end of the study there was a significant increase in the use of PAH therapy and combination therapy but was not different between groups.
There were no significant differences in the distribution of NYHA functional class between groups at baseline or last clinic visit ( Figure 2 ). At baseline 53% of patients were in functional class III or IV. The percentage of patients who developed a worsening NYHA functional class over the period of study also was not significantly different between groups.

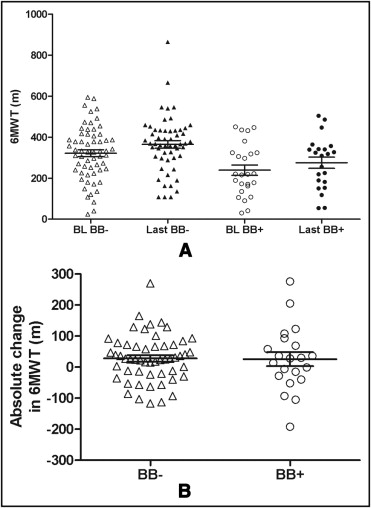
Baseline 6MWT was significantly lower in the β-blocker group (239 ± 127 m) compared to the no–β-blocker group (322 ± 131 m, p = 0.009; Figure 3 ). Over the study period 6MWT increased similarly in the 2 groups (26 ± 100 vs 28 ± 75 m in the β-blocker vs no–β-blocker group respectively, p = 0.92; Figure 3 ).
As presented in Table 3 , all baseline echocardiographic parameters were similar between the 2 groups. A significant proportion of patients in the 2 groups had moderate or severe RV dysfunction (39%, assessed qualitatively by echocardiography) and moderate or severe tricuspid regurgitation (57%). Prevalence of moderate or severe RV dysfunction tended to improve over time but was not different between groups at the end of the study.
| Parameters | No Beta-Blocker | Beta-Blocker | ||
|---|---|---|---|---|
| Baseline | Last | Baseline | Last | |
| (n = 68) | (n = 60) | (n = 26) | (n = 20) | |
| Normal left ventricular function | 63 (93%) | 57 (95%) | 24 (92%) | 19 (95%) |
| Right atrial dilation | 37 (63%) | 40 (67%) | 18 (78%) | 16 (89%) |
| Right ventricular dilation | 31 (48%) | 29 (50%) | 14 (61%) | 12 (63%) |
| Moderate/severe right ventricular dysfunction | 25 (37%) | 19 (33%) | 12 (46%) | 4 (21%) |
| Moderate/severe tricuspid regurgitation | 38 (56%) | 25 (47%) | 16 (62%) | 8 (62%) |
| Pericardial effusion | 13 (19%) | 9 (15%) | 4 (15%) | 2 (10%) |
| Right ventricular systolic pressure (mm Hg), mean ± SD | 74.8 ± 17.8 | 70.3 ± 23.9 | 68.6 ± 21.2 | 69.6 ± 20.8 |
Right heart catheterizations were done at the baseline visit in 80% of study patients, and 36% of patients had a second clinically indicated right heart catheterization during the study period. All baseline hemodynamic parameters were similar; however, the β-blocker group had a lower heart rate (p = 0.0001; Table 1 ) and higher median right atrial pressure (p = 0.047; Table 4 ). Over time the 2 groups developed a similar slight decrease in mean pulmonary artery pressure and pulmonary vascular resistance. However, the cardiac index increased to a greater degree in the β-blocker group (absolute increase 0.9 ± 0.4 vs 0.4 ± 0.6 L/min/m 2 in the β-blocker vs no–β-blocker group, respectively, p = 0.027).
| Parameters | No Beta-Blocker | Beta-Blocker | ||
|---|---|---|---|---|
| Baseline | Last | Baseline | Last | |
| (n = 55) | (n = 20) | (n = 20) | (n = 7) | |
| Systolic pulmonary arterial pressure (mm Hg) | 70.2 ± 21.5 | 65.2 ± 24.6 | 75.4 ± 17.8 | 72.6 ± 15.1 |
| Mean pulmonary arterial pressure (mm Hg) | 42.4 ± 12.4 | 41.2 ± 13.7 | 45.7 ± 11.8 | 42 ± 11.9 |
| Mean pulmonary capillary wedge pressure (mm Hg) | 9.4 ± 4.0 | 9.9 ± 4.3 | 10.9 ± 3.4 | 9.4 ± 3.3 |
| Mean right atrial pressure (mm Hg) | 6.8 ± 4.2 | 6.7 ± 5.0 | 9.3 ± 5.9 ⁎ | 8.9 ± 5.1 |
| Pulmonary arterial oxygen saturation (%) | 64.6 ± 7.8 | 67.6 ± 9.9 | 61.2 ± 9.5 | 68.5 ± 5.0 |
| Cardiac output by Fick (L/min) | 4.1 ± 1.2 | 4.8 ± 1.1 | 3.6 ± 0.6 | 5.2 ± 0.9 |
| Cardiac index by Fick (L/min/m 2 ) | 2.3 ± 0.6 | 2.7 ± 0.6 | 1.9 ± 0.4 † | 2.8 ± 0.4 |
| Pulmonary vascular resistance (Woods unit) | 8.7 ± 4.2 | 7.1 ± 4.7 | 10.0 ± 4.9 | 5.7 ± 2.2 |
Clinical event rates were high in the overall cohort; however, statistically significant differences were not observed between groups ( Table 5 ). All-cause mortality or PAH-related hospitalization was not different between groups. The percentage of subjects who had ≥1 adverse event (death or PAH-related hospitalization) was slightly larger in the β-blocker group (50%) compared to the no–β-blocker group (35%) but was not statistically significantly different (p = 0.19).



