Percutaneous ventricular assist devices (PVADs) and intraaortic balloon pump (IABP) are used to provide mechanical circulatory support (MCS) for high-risk percutaneous coronary intervention (PCI). Contemporary trends in their utilization and impact on in-hospital mortality are not known. Using the National Inpatient Sample (2004 to 2012), we identified 5,031 patients who received a PVAD and 122,333 who received an IABP on the same day as PCI using International Classification of Diseases, Ninth Edition codes. Utilization of MCS increased from 1.3% of all PCIs in 2004 to 3.4% in 2012 (p trend <0.001), with increase in the use of both PVAD (<1/10,000 PCIs [2004 to 2007] to 38/10,000 [2012]) and IABP (132/10,000 PCIs [2004] to 299/10,000[2012] p <0.0001 for both). PVAD recipients were older (69 vs 65 years), more likely to have heart failure (68% vs 41%), chronic kidney disease (27% vs 11%, p <0.001 for all), and be admitted electively (30% vs 11%), but less likely to have acute myocardial infarction (52% vs 90%), cardiogenic shock (23% vs 50%), or need mechanical ventilation (16% vs 29%) compared with IABP recipients. Unadjusted in-hospital mortality was lower in PVAD compared with IABP recipients (12.8% vs 20.9%, p <0.001). However, in propensity-matched analyses (1:2), in-hospital mortality was similar in both groups (odds ratio 0.88, 95% confidence interval 0.70 to 1.09). In conclusion, there has been a marked increase in the utilization of MCS in patients undergoing PCI. Unadjusted mortality using PVADs is lower than IABP but may be due to their selective use in patients at lower risk. Randomized trials are necessary to establish their effectiveness in supporting high-risk PCI.
Nearly 1 million patients undergo percutaneous coronary intervention (PCI) in the United States each year, of which 18% are considered high risk. The risk of mortality is nearly twofold greater in patients undergoing high-risk PCI compared to other patients with PCI. The use of mechanical circulatory support (MCS) devices is supported by clinical practice guidelines in patients undergoing high-risk PCI procedures. Until recently, the choice of MCS was limited to intraaortic balloon pump (IABP). However, percutaneous ventricular assist devices (PVADs), namely the Impella and the TandemHeart, which provide superior hemodynamic support compared to IABP are increasingly used in clinical practice. Although expert consensus on utility of MCS devices in high-risk PCI, empirical data regarding the comparative effectiveness of MCS in patients undergoing high-risk PCI are lacking. Despite limited evidence, the use of MCS devices especially PVADs has increased in recent years. We used the National Inpatient Sample (NIS) data, a large national administrative database of hospitalized patients, to examine contemporary use of MCS on the same day as a PCI procedure. We examined national trends in the use, clinical characteristics, and patient outcomes in patients receiving MCS devices on the same day as PCI, and compared the use of PVAD with IABP in this setting.
Methods
The NIS is the largest all-payer database of hospitalized patients in the United States managed under the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality. The design of the NIS has been described previously and has been included in the Appendix . Briefly, the NIS is comprised a random 20% sample of patients hospitalized at acute care hospitals from participating states during a given year, obtained after stratification of hospitals by bed size, teaching status and census region. Patient-level and hospital-level weights were provided to obtain national estimates. The NIS includes detailed information on patient characteristics including demographic variables (e.g., age, gender, race, and so on) and primary and secondary discharge diagnoses and procedures. Both diagnoses and procedures are available as International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9 CM) codes and their validated combinations into broad categories using the Clinical Classification of Diseases Software (CCS). We used a combination of ICD-9 and CCS codes to identify co-morbid conditions and inpatient procedures. A maximum of 25 discharge diagnoses and 15 procedures can be included for each patient. For each procedure, the day of the procedure is also available. Given that PVAD procedures were first included in the NIS in the year 2004, we restricted our study cohort to years 2004 to 2012.
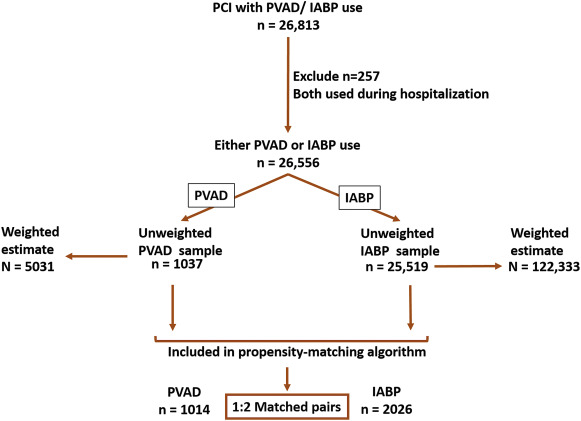
We identified all patients, aged 18 years and older, who underwent PCI (CCS code: 45) during 2004 to 2012 along with placement of either a PVAD (ICD-9 CM code, 37.68) or an IABP (ICD-9 CM code, 37.61) on the same day as the PCI procedure. Patients in whom both PVAD and IABP were used in the same hospitalization were excluded (n = 257). Figure 1 shows a schematic diagram of patient selection.
First, we examined calendar year trends in the use of PCI procedures supported by either PVAD or IABP. Second, we compared patient demographics, co-morbidities, discharge disposition (e.g., home, home with home health, and other health care institution), and length of stay for PVAD and IABP groups using the Rao–Scott chi-square test for categorical variables and survey-specific paired t test for continuous variables. Because length of stay was not normally distributed, we transformed that variable to its natural logarithm and compared the geometric mean of the transformed length of stay variables between the 2 groups. Next, we compared unadjusted in-hospital mortality using similar methods as mentioned previously. All descriptive analyses are used to obtain unbiased national estimates of outcome frequencies, means, and variances while accounting for the survey design.
To examine the association between the type of MCS device (PVAD vs IABP) and in-hospital mortality, we conducted a matched propensity score analysis to explicitly account for confounding by indication (selection bias). In our model, which explicitly accounted for the survey design of the NIS, we matched each PVAD recipient to 2 IABP recipients with similar covariates, represented as propensity scores. Variables used in our model for estimating propensity scores included age, gender, race, discharge diagnoses (cardiogenic shock, acute myocardial infarction (AMI), cardiac arrest, previous coronary atherosclerosis, heart failure, valvular heart disease, cardiac arrhythmias, and peripheral arterial disease), co-morbidities (diabetes, hypertension, dyslipidemia, cancer, liver disease, chronic kidney disease (CKD), fluid-electrolyte disorder, chronic obstructive pulmonary disease, coagulopathy, and substance use disorder), procedures (coronary artery bypass graft surgery or mechanical ventilation), and nature of admission (nonelective vs elective). Details of the propensity matching algorithm are included in the Appendix . To test whether our matching algorithm was successful in achieving covariate balance, we calculated standardized differences for all covariates and compared them between PVAD and IABP groups before and after matching. A standardized difference of <10% for all covariates after matching is indicative of a successful match. We used the Cochran–Mantel–Haenszel test for matched data to compare the effect of PVAD with IABP on in-hospital mortality.
Given that the risk of mortality in patients undergoing PCI differs according to the level of acuity, we repeated our analyses previously mentioned to examine mortality separately in patients with (1) cardiogenic shock, (2) AMI without cardiogenic shock, and (3) without AMI or cardiogenic shock. The subgroup of patients without AMI or cardiogenic shock likely represents patients undergoing high-risk elective PCI, given the absence of acute indications for hemodynamic support (i.e., AMI and cardiogenic shock). For comparative and subgroup analysis, we used domain analysis which ensures that the assumption of an infinite discharge universe is satisfied (central to the analysis of the NIS data) and that the estimated population statistics and measurements of variance are accurate.
We performed sensitivity analyses to assess the robustness of our findings. First, to account for unmeasured confounding due to differences in hospitals with and without PVAD programs, we restricted IABP recipients to those hospitals where at least 1 PVAD device was used in a given year. Second, to account for the availability of Impella devices, we limited the analysis to years 2009 to 2012 to capture the years after the approval of the first Impella device (Impella 2.5 Recover; Abiomed, Danvers, MA) in May 2008.
The level of significance was set at a p value of 0.05. All analyses were performed using SAS 9.4 software (SAS institute, Cary, North Carolina), including SAS PROC SURVEYMEANS and PROC SURVEYFREQ, for analysis of complex survey data. The study was reviewed by the University of Iowa Institutional Review Board, which waived the requirement for informed consent.
Results
During 2004 to 2012, we identified 1,037 patients who received a PVAD and 25,519 patients who received an IABP on the same day as PCI in the NIS data, which translate to an estimated 5,031 PVAD and 122,333 IABP procedures in the United States during this period ( Figure 1 ). Overall, a percutaneous MCS device (PVAD or IABP) was used in 2.1% of all patients with PCI. Within subgroups, a percutaneous MCS device was used in 40.7% of patients with cardiogenic shock and 2.2% of patients with AMI and no cardiogenic shock. Utilization of MCS increased from 1.3% of all PCIs in 2004 to 3.4% in 2012 (p for trend <0.0001) with simultaneous increase in utilization of both PVADs (<1 device per 10,000 PCIs during 2004 to 2007 to 38 devices per 10,000 PCIs in 2012, p for trend <0.0001) and IABP (132 devices per 10,000 PCIs in 2004 to 299 devices per 10,000 PCIs in 2012, p for trend <0.0001; Figure 2 ). The increase in utilization of MCS devices occurred within subgroups of patients with cardiogenic shock, patients with AMI and without cardiogenic shock as well as patients without cardiogenic shock or AMI ( Figure 3 ).
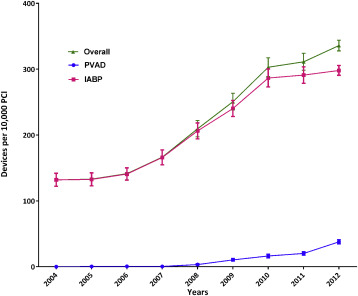
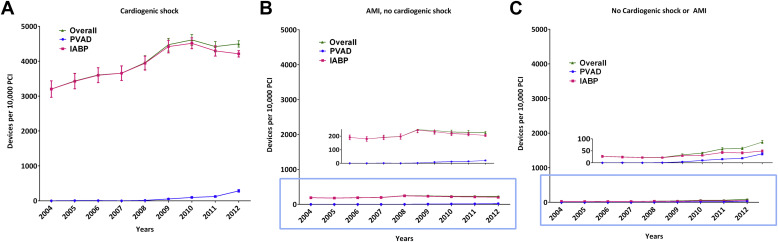
Table 1 summarizes differences in patients who received PVAD compared with IABP on the same day as PCI. Patients who received PVAD were more likely to be older, men, and have congestive heart failure, CKD, hypertension, and diabetes but less likely to have AMI, cardiogenic shock, cardiac arrest, undergo coronary artery bypass graft surgery, or require mechanical ventilation (p <0.0001 for all, Table 1 ) compared with IABP recipients. Although length of stay was significantly longer in patients with PVAD, the magnitude of difference was small (8.3 vs 7.8 days). Compared with IABP recipients, patients who received PVAD on the same day as PCI had lower unadjusted in-hospital mortality (20.9% in IABP vs 12.8% in PVAD, p <0.0001; Table 2 ).
| Characteristics | PVAD | IABP | p-value |
|---|---|---|---|
| Estimated number (N) ∗ | 5031 (351) | 122333 (4661) | |
| Mean Age in years (SEM) | 69.0 (0.4) | 64.7 (0.1) | <0.0001 |
| Age ≥ 65 years | 64.6% (1.5%) | 50.3% (0.5%) | <0.0001 |
| Male Sex | 74.1% (1.3%) | 69.0% (0.3%) | 0.0002 |
| Race | 0.0243 | ||
| White | 65.7% (2.0%) | 67.4% (1.1%) | |
| Black | 9.1% (1.2%) | 6.1% (0.3%) | |
| Others | 16.2% (1.6%) | 14.9% (0.7%) | |
| Missing/unknown | 8.9% (1.7%) | 11.6% (1.1%) | |
| Income quartiles † | 0.0019 | ||
| 0-25 | 32.9% (2.1%) | 26.3% (1.0%) | |
| 26 to 50 | 25.3% (1.5%) | 25.0% (0.8%) | |
| 51 to 75 | 21.8% (1.5%) | 24.5% (0.6%) | |
| 76 to 100 | 20.1% (2.1%) | 24.2% (1.4%) | |
| Discharge diagnoses/procedures | |||
| Acute myocardial infarction | 52.5% (1.7%) | 89.7% (0.4%) | <0.0001 |
| Cardiogenic shock | 23.3% (1.7%) | 49.6% (0.9%) | <0.0001 |
| Heart failure | 68.5% (1.7%) | 41.3% (0.6%) | <0.0001 |
| Cardiac arrest | 12.2% (1.1%) | 24.7% (0.4%) | <0.0001 |
| Valvular heart disease | 21.6% (1.4%) | 12.7% (0.3%) | <0.0001 |
| Arrhythmia | 38.4% (1.5%) | 40.6% (0.4%) | 0.16 |
| Prior coronary artery disease ‡ | 95.2% (0.7%) | 85.2% (0.5%) | <0.0001 |
| Hypertension | 70.6% (1.6%) | 55.8% (0.6%) | <0.0001 |
| Diabetes Mellitus | 46.5% (1.7%) | 32.3% (0.5%) | <0.0001 |
| Dyslipidemia § | 57.8% (1.6%) | 46.1% (0.7%) | <0.0001 |
| Chronic kidney disease | 27.2% (1.5%) | 11.0% (0.3%) | <0.0001 |
| Fluid/electrolyte disorder | 26.4% (1.6%) | 28.8% (0.6%) | 0.16 |
| Chronic obstructive pulmonary disease | 19.8% (1.3%) | 14.1% (0.3%) | <0.0001 |
| Liver disease | 8.7% (1.0%) | 7.7% (0.3%) | 0.33 |
| Cancer | 9.3% (0.9%) | 7.0% (0.2%) | 0.005 |
| Coagulation disorder | 9.5% (1.0%) | 10.6% (0.3%) | 0.34 |
| Substance-abuse | 1.2% (0.3%) | 1.7% (0.1%) | 0.0310 |
| Coronary bypass | 1.3% (0.3%) | 13.0% (0.4%) | <0.0001 |
| Mechanical ventilation | 15.9% (1.2%) | 28.6% (0.7%) | <0.0001 |
| Disposition | <0.0001 | ||
| Home or self-care | 56.3% (1.7%) | 48.6% (0.8%) | |
| Short term hospital | 1.3% (0.4%) | 5.2% (0.3%) | |
| Skilled care facility | 15.8% (1.3%) | 12.9% (0.3%) | |
| Home health care | 13.6% (1.2%) | 12.0% (0.3%) | |
| Against medical advice or unknown | 0.2% (0.1%) | 0.4% (0.04%) | |
| Died | 12.8% (1.2%) | 20.9% (0.5%) | |
| Payment source | <0.0001 | ||
| Medicare | 65.7% (1.7%) | 49.2% (0.5%) | |
| Medicaid | 7.2% (0.8%) | 7.2% (0.3%) | |
| Private insurance | 21.5% (1.5%) | 32.9% (0.5%) | |
| Others | 5.6% (0.8%) | 10.7% (0.3%) | |
| Elective admission | 29.6% (2.0%) | 11.4% (0.5%) | <0.0001 |
| Length of stay in days (SEM) | 7.8 (0.3) | 8.3 (0.1) | <0.0001 |
| In-hospital mortality | 12.8% (1.2%) | 20.9% (0.5%) | <0.0001 |
∗ Estimated numbers obtained after application of patient-level discharge weights.
† Median household income quartiles based on patient zip code.
‡ Previous coronary artery disease (CCS code 101), includes patients with previous documented coronary disease, represented by the history of coronary bypass, previous PCI, previous myocardial infarction or angina pectoris. It does not include acute myocardial infarction in index hospitalization.
§ Dyslipidemia (CCS code 53), includes any of these, hypercholesterolemia (ICD-9 code 272.0), hypertriglyceridemia (272.1), mixed hyperlipidemia (272.2), hyperchylomicronemia (272.3), and other hyperlipidemia (272.4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


