Evolocumab (AMG 145), a fully human monoclonal antibody against PCSK9, significantly reduced low-density lipoprotein cholesterol (LDL-C) levels in phase 2 and 3 studies. This phase 3 study evaluated the efficacy and safety of evolocumab plus atorvastatin in Japanese patients with hyperlipidemia or mixed dyslipidemia and high cardiovascular risk. Patients were randomized to atorvastatin 5 or 20 mg/day for 4 weeks. Subsequently, patients underwent second randomization to evolocumab 140 mg biweekly (Q2W) or 420 mg monthly (QM) or placebo Q2W or QM. Coprimary end points were % change from baseline in LDL-C at week 12 and mean of weeks 10 and 12. Secondary end points included change and % change in other lipids and proportion of patients reaching LDL-C <70 mg/dl. Adverse events and laboratory values were recorded. Four hundred four patients were randomized to study drug. At baseline, the mean (SD) age was 61 (10) years (placebo) and 62 (11) years (evolocumab); 39% and 40% were women; 14% and 12% had cerebrovascular or peripheral arterial disease; and 51% and 47% had diabetes. At entry, mean (SD) calculated LDL-C was 128 (23) mg/dL; after stabilization on atorvastatin 5 and 20 mg/day, baseline LDL-C levels were 118 (35) and 94 (24) mg/dL, respectively. Mean LDL-C reductions at week 12 for evolocumab versus placebo ranged from 67% to 76%. No imbalances were observed in adverse events between treatment groups. Efficacy and safety for Q2W or QM evolocumab dosing were similar. In conclusion, in high-risk Japanese patients receiving stable statin therapy, evolocumab markedly reduced LDL-C and was well tolerated.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a serine protease involved in the regulation of low-density lipoprotein (LDL) receptor expression. PCSK9 binds to the LDL receptor and targets it for destruction, leading to decreased LDL receptor expression and an increase in plasma LDL cholesterol (LDL-C). Inhibiting PCSK9 with a monoclonal antibody reduced serum LDL-C levels in preclinical studies. Evolocumab is a fully human monoclonal immunoglobulin G2 antibody against PCSK9 that markedly reduced serum LDL-C levels in phase 2 and 3 studies. In the first phase 2 study reported of a PCSK9 inhibitor in patients in Japan, evolocumab plus background statin therapy reduced LDL-C levels by up to 69% from baseline versus placebo; favorable changes were seen in other lipid parameters, including lipoprotein (a) (Lp[a]) reductions of up to 51% versus placebo. YUKAWA-2 (Study of LDL-Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk; NCT01953328 ) is the first phase 3 and largest clinical study to date of a PCSK9 inhibitor in Japan. The primary objective of the study was to evaluate the efficacy of 12 weeks of subcutaneous evolocumab administered biweekly (Q2W) or monthly (QM) in combination with atorvastatin in Japanese patients with hyperlipidemia or mixed dyslipidemia and at high cardiovascular (CV) risk.
Methods
Eligible patients (aged ≥20 and ≤85 years) from study sites in Japan were at high risk for CV events based on Japan Atherosclerosis Society (JAS) criteria. JAS criteria used for high-risk classification can be found in the Supplementary Data . Patients were required to be on a stable dose of an approved statin for ≥4 weeks before LDL-C screening without need for uptitration; use of any other lipid-lowering therapy had to be unchanged within 4 weeks before LDL-C screening. At screening (before randomization to study-specified background statin therapy), fasting LDL-C level was required to be ≥100 mg/dl (2.6 mmol/L), and fasting triglyceride level was required to be ≤400 mg/dl (4.5 mmol/L). Key exclusion criteria can be found in the Supplementary Data . Independent ethics committees at each study site approved the protocol, and all patients provided written informed consent before initiation of study-specific procedures.
Before randomization, all eligible patients underwent a placebo run-in period (3 placebo injections) to confirm tolerance of subcutaneous injections. Patients were then randomized 1:1 to 1 of 2 atorvastatin treatment groups consistent with low (5 mg/day) and high (20 mg/day) statin doses used in clinical practice in participating regions. After successful completion of a 4-week lipid stabilization period, patients in each atorvastatin dose cohort were further randomized 1:1:1:1 to 1 of 4 treatment groups: placebo Q2W, placebo QM, evolocumab 140 mg Q2W, or evolocumab 420 mg QM. Patients were randomized to atorvastatin therapy in 3 strata: patients diagnosed with heterozygous familial hypercholesterolemia (HeFH); patients without HeFH receiving intensive lipid-lowering therapy (defined according to local treatment practices as atorvastatin ≥10 mg/day or equivalent); and patients without HeFH receiving nonintensive lipid-lowering therapy. Patients, investigators, and study site personnel were blinded to treatment.
The co-primary efficacy end points were the mean % change from baseline in LDL-C at week 12 (end of study) and the mean % change from baseline in LDL-C at weeks 10 and 12 (time-averaged reduction in LDL-C). Secondary end points included the mean change from baseline at weeks 10 and 12 and at week 12 in LDL-C; the mean % change from baseline at weeks 10 and 12 and at week 12 in other lipids, including apolipoprotein B (ApoB); achievement of LDL-C <70 mg/dl; and % change from baseline in Lp(a), triglycerides, high-density lipoprotein cholesterol (HDL-C), and very-low-density lipoprotein cholesterol. When calculated LDL-C was <40 mg/dl or triglyceride was >400 mg/dl, a value from the same blood sample measured through preparative ultracentrifugation was used, if available.
A sample size of 45 patients in each of the 8 treatment groups was calculated to provide >90% power to detect the treatment effect (30% reduction in LDL-C with a common SD of 30%) of evolocumab compared with placebo. For the coprimary end points, a repeated measures linear effects model was used for each atorvastatin dose cohort and dose frequency to compare the efficacy of evolocumab with placebo. The repeated measures model included terms for treatment group, stratification factor, scheduled visit, and interaction of treatment with scheduled visit. No imputation of missing data was performed for the repeated measures linear effects model. Analyses for the secondary end points were adjusted for multiplicity conditional on the primary end point meeting statistical significance. The secondary end point of LDL-C response at week 12 was analyzed using the Cochran–Mantel Haenszel test adjusted by stratification factor. Safety outcomes included the incidences of treatment-emergent adverse events (AEs) and serious AEs (SAEs), treatment-related AEs, AEs leading to discontinuation of investigational product, and laboratory parameters. AEs were coded using the Medical Dictionary for Regulatory Activities, version 17.0, and graded by severity using the Common Terminology for Adverse Events (CTCAE), version 4.3. Laboratory analyses were based on CTCAE, version 4, toxicity criteria.
Results
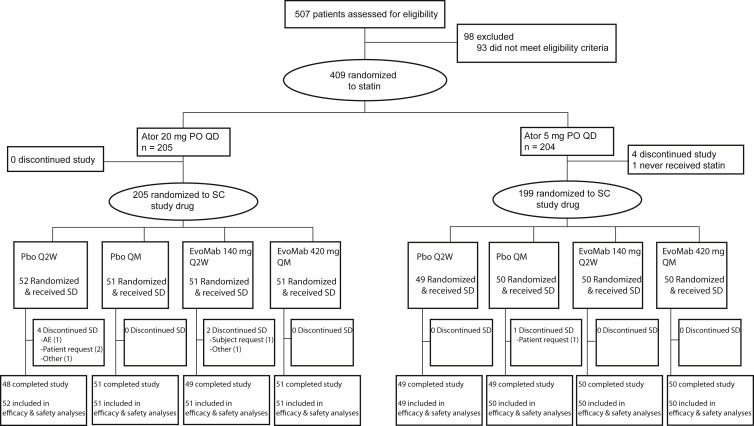
In total, 507 patients were screened for the study and 409 were randomized to 1 of 2 atorvastatin doses (5 mg/day or 20 mg/day). All patients were able to tolerate the 3 placebo injections given to assess tolerability (none failed to enter the study because of intolerance of placebo injections). A total of 404 patients successfully completed the 4-week lipid stabilization period and were subsequently randomized and received study drug ( Figure 1 ). The first patient enrolled on October 7, 2013, and the last patient last visit was June 25, 2014. In general, baseline patient characteristics were balanced between the placebo and evolocumab treatment groups ( Table 1 ). To be eligible for participation in this study, all patients were required to be at high CV risk based on local guidelines. Patients most often satisfied this criteria by having diabetes, possessing 2 or more CV risk factors (such as advanced age, hypertension, or smoking history) or by being a secondary prevention patient. At baseline, half of patients had a diagnosis of type 2 diabetes mellitus and one quarter had a diagnosis of metabolic syndrome. Twenty-eight percent of placebo-treated and 37% of evolocumab-treated patients with metabolic syndrome at baseline had elevated fasting plasma glucose (≥110 mg/dl). Study eligibility criteria required all patients to have received stable statin therapy (defined as ≥4 weeks) at home. At screening, 100% of patients were on a statin. The mean (SD) LDL-C level before the initiation of treatment with study-provided atorvastatin was 128 (23) mg/dl. In patients randomized to background atorvastatin 5 mg/day, the mean (SD) baseline LDL-C at the end of the lipid stabilization period but before study drug administration was 115 (28) mg/dl in the placebo group and 120 (41) mg/dl in the evolocumab group. In patients on background atorvastatin 20 mg/day, the corresponding values were 91 (23) mg/dl in the placebo group and 97 (25) mg/dl in the evolocumab group.
| Variable | Placebo + Atorvastatin N = 202 | Evolocumab + Atorvastatin N = 202 |
|---|---|---|
| Age (years, mean ± SD) | 61 ± 10 | 62 ± 11 |
| Women | 79 (39%) | 81 (40%) |
| Coronary artery disease | 22 (11%) | 30 (15%) |
| Peripheral arterial disease or cerebrovascular disease | 29 (14%) | 25 (12%) |
| Hypertension | 145 (72%) | 152 (75%) |
| Elevated waist circumference | 138 (68%) | 138 (68%) |
| Current smoker | 52 (26%) | 46 (23%) |
| Heterozygous familial hypercholesterolemia | 11 (5%) | 13 (6%) |
| Simon-Broome | 5 (3%) | 3 (2%) |
| Other ∗ | 6 (3%) | 10 (5%) |
| Type 2 diabetes mellitus | 103 (51%) | 94 (47%) |
| Metabolic syndrome † | 57 (28%) | 54 (27%) |
| ≥ 2 cardiovascular risk factors | 117 (58%) | 113 (56%) |
| LDL-C (mg/dL, mean ± SD) ‡ | 103 ± 28 | 109 ± 35 |
| Lipoprotein (a) (nmol/L, median [Q1, Q3]) ‡ | 31 (12, 50) | 34 (14, 61) |
| HDL-C (mg/dL,mean ± SD) ‡ | 58 ± 14 | 56 ± 14 |
| Triglycerides (mg/dL, median [Q1, Q3]) ‡ | 127 (91, 168) | 118 (92,155) |
| Apolipoprotein B (mg/dL, mean ± SD) ‡ | 92 ± 20 | 96 ± 25 |
| PCSK9 (ng/mL, mean [SD]) ‡ | 356 (99) | 362 (100) |
∗ Japanese definition based on criteria from Harada-Shiba et al 2012 (reference 43).
† Metabolic syndrome was defined as 3 or more risk factors (elevated waist circumference, elevated systolic blood pressure or diastolic blood pressure, elevated fasting glucose, elevated triglycerides, and/or low HDL-C) and without diabetes mellitus.
‡ Collected after the lipid-stabilization period and before administration of first dose of study drug.
At week 12, evolocumab reduced LDL-C by ≥67% versus placebo in all evolocumab treatment groups, with mean reductions ranging from 67% to 76% ( Table 2 ; Figure 2 ). Reductions in LDL-C at the time-averaged mean of weeks 10 and 12 were comparable with those observed at week 12 (data not shown). Efficacy in the 140-mg Q2W and 420-mg QM doses was comparable; reductions were also comparable between patient subgroups, including age, gender, body mass index (BMI), type 2 diabetes mellitus, and metabolic syndrome ( Supplementary Figure S1 ).
| Variable | Evolocumab + Atorvastatin 5 mg/d | Evolocumab + Atorvastatin 20 mg/d | ||
|---|---|---|---|---|
| Q2W (N = 50) | QM (N = 50) | Q2W (N = 51) | QM (N = 51) | |
| LS mean (SE) treatment difference versus placebo + atorvastatin | ||||
| Low-density lipoprotein cholesterol | –74.9% (2.7) ∗ | –69.9% (2.4) ∗ | –75.9% (3.9) ∗ | –66.9% (3.0) ∗ |
| Apolipoprotein B | –65.6% (2.4) ∗ | –57.2% (2.4) ∗ | –60.4% (2.8) ∗ | –56.2% (2.4) ∗ |
| High-density lipoprotein cholesterol | 13.5% (3.1) ∗ | 15.2% (2.7) ∗ | 16.9% (3.1) ∗ | 10.2% (2.7) ∗ |
| Lipoprotein (a) | –50.1% (7.6) ∗ | –48.8% (5.9) ∗ | –52.7% (5.7) ∗ | –40.0% (5.3) ∗ |
| Triglycerides | –27.6% (9.5) † | –20.0% (5.9) ∗ | –17.2% (5.5) † | –16.9% (7.2) ‡ |
| Apolipoprotein A1 | 7.3% (2.4) † | 8.9% (2.2) ∗ | 9.1% (2.4) ∗ | 8.6% (2.3) ∗ |
| Achieved low-density lipoprotein cholesterol | ||||
| Median (Q1, Q3) Achieved LDL-C, mg/dL | 26.0 (16.0, 40.0) | 36.0 (29.0, 43.0) | 24.5 (18.0, 34.0) | 26.0 (20.0, 38.0) |
| Low-density lipoprotein cholesterol < 70 mg/dL, % patients § | 98.0% | 96.0% | 96.0% | 98.0% |
§ At week 12, in patients receiving 5 mg/day atorvastatin, 0% placebo Q2W and 4% placebo QM reached LDL-C < 70; in patients receiving 20 mg/day atorvastatin, 20% placebo Q2W and 20% placebo QM reached LDL-C < 70.
The median (first quartile, third quartile) achieved LDL-C level at week 12 for patients receiving evolocumab plus atorvastatin was 28 (20, 40) mg/dl and for patients receiving placebo plus atorvastatin was 97 (83, 115) mg/dl. The 2012 JAS guidelines recommend LDL-C <120 or <100 mg/dl for patients at high risk or secondary prevention, respectively. In patients receiving placebo plus atorvastatin, 29% on Q2W placebo plus atorvastatin 5 mg/day and 42% on QM placebo plus atorvastatin 5 mg/day reached an LDL-C <100 mg/day. Most patients receiving placebo plus atorvastatin 20 mg/day were able to achieve an LDL-C <100 mg/dl (59% placebo Q2W and 88% placebo QM). Few patients were able to achieve a more aggressive target LDL-C of <70 mg/dl on placebo plus atorvastatin therapy (0% placebo Q2W plus atorvastatin 5 mg/day and 4% placebo QM plus atorvastatin 5 mg/day; 20% placebo Q2W plus atorvastatin 20 mg/day and 20% placebo QM plus atorvastatin 20 mg/day). In contrast, 100% of patients treated with evolocumab achieved an LDL-C <100 mg/dl, and almost all achieved an LDL-C level <70 mg/dl, regardless of atorvastatin dose ( Table 2 , Figure 3 ).
Favorable changes were observed in other lipid parameters at week 12 in the evolocumab treatment groups ( Table 2 ; Figure 4 ) with results being similar to the time-averaged mean of weeks 10 and 12 (data not shown). The changes were comparable between 140-mg Q2W and 420-mg QM doses. Across evolocumab treatment groups, the mean (SE) treatment differences versus placebo at week 12 ranged from −56% (2%) to −66% (2%) for apolipoprotein B; 10% (3%) to 17% (3%) for HDL-C; and −40% (5%) to −53% (6%) for Lp(a).
AEs were comparable between patients receiving placebo and those receiving evolocumab ( Table 3 ) without notable differences seen between patients receiving 140-mg Q2W or 420-mg Q2W (data not shown). Patients demonstrated similar rates of discontinuation and AEs (including injection site reactions) across all placebo and evolocumab treatment groups, suggesting good tolerability of both Q2W and QM dosing regimens. The most common AEs occurring in ≥2% of patients in any treatment group are listed in Table 3 . SAEs were infrequent in both groups. One placebo-treated patient discontinued study drug because of an SAE (brain stem infarction). Transaminase and creatine kinase elevations were infrequent ( Table 3 ). Few patients experienced injection site reactions; all were mild, CTCAE grade 1 ( Table 3 ). One patient treated with evolocumab with a negative or no result at baseline was found to have an antievolocumab-binding antibody ( Table 3 ); this patient completed the study and had 3 nonserious AEs of upper respiratory tract infection (twice) and herpes labialis (once); none was deemed related to evolocumab. No patient reported neurocognitive AEs. Changes at week 12 in fasting plasma glucose and glycated hemoglobin (HbA 1c ) were not notably different in patients receiving evolocumab versus those receiving placebo ( Supplementary Table S1 ). In addition, rates of AEs and laboratory abnormalities were comparable between patients with low LDL-C (<15 mg/dl, <25 mg/dl or <40 mg/dl; all patients in these groups were treated with evolocumab) and those with higher LDL-C (≥40 mg/dl; all placebo patients and 11 patients treated with evolocumab; Supplementary Table S2 ).



