Chapter 76 Use of Ablation to Treat Arrhythmias in Children and Patients with Congenital Heart Disease
Transcatheter Ablation of Arrhythmias in Children with Structurally Normal Hearts
Supraventricular tachycardia (SVT) accounts for the majority of childhood arrhythmias. Re-entrant tachycardia secondary to accessory pathways (APs) is responsible for 75% of SVTs in children with AV nodal re-entrant tachycardia and primary atrial tachycardia accounting for the rest.1 Ventricular tachycardia is rare (only 5% of all tachycardias).2
The overall acute success for AP ablation is 91%, with higher success for left-sided pathways (96%) versus septal pathways (87%) and right free wall pathways (86%).3,4 These results may be even better with the lower risk of complications such as heart block in the present era since the advent of three-dimensional mapping and cryo ablation. The risk of major complications in the Pediatric Electrophysiology Registry comprising 4651 procedures was 3.2% and included AV block, perforation, pericardial effusion, brachial plexus injury because of arm position, emboli, and pnemothorax.4,5–7 Body weight of 15 kg or less correlated with a higher complication rate in one study.4 The overall death rate was 0.15%, which was similar to that reported for adult ablations.3
Indications for radiofrequency (RF) ablation for cardiac arrhythmias in children are as follows:5
Procedural Implications
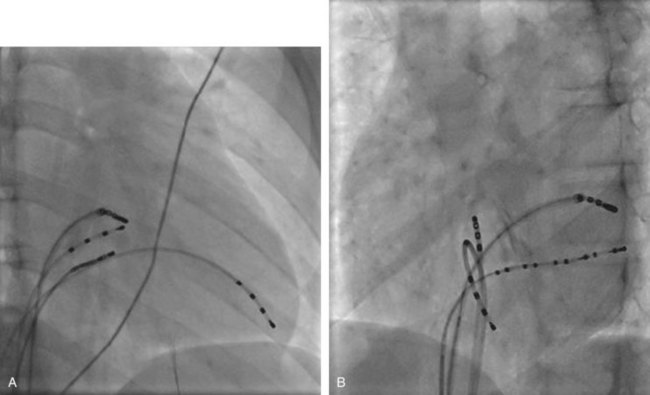
The ablation procedure, including catheter placement, programmed electrical stimulation, and recording and analysis of electrograms, is similar to that in adults. Standard CA includes placement of a multiple-polar (octapolar or decapolar) catheter in the coronary sinus from either the right internal jugular vein or the femoral vein and quadripolar catheters in the His bundle and right ventricular apex positions (Figure 76-1). Programmed electrical stimulation (PES) and pacing maneuvers are performed from the atrium and the ventricle to identify the location and characteristics of the AP (such as effective refractory period [ERP]) and inducibility of different arrhythmias. The procedure can be modified by using smaller caliber and fewer catheters in younger patients without compromising the procedure. This can be accomplished by the occasional use of an esophageal electrode for atrial stimulation and recording to replace an intracardiac catheter or by the use of His–right ventricular apex (RVA) multiple-polar catheter to obtain both RVA and His-bundle signals with a single catheter. Attention must be paid to the procedure, anesthesia duration, and fluoroscopy times, as younger patients are more susceptible to radiation effects. Ideally, pediatric interventional electrophysiology laboratories need to be specifically equipped with bi-plane, low-dose-rate pulsed fluoroscopy, and special shielding techniques. Availability of pediatric cardiac anesthesiologists, pediatric interventional cardiologists, and specially trained pediatric nursing personnel are essential to the safety and success of electrophysiology procedures in children.
Sedation and Anesthesia
In children older than 10 years of age who are not overly anxious or resistant to intravenous (IV) sedatives, conscious sedation and careful monitoring may be used. General anesthesia, with or without endotracheal intubation, may be necessary in children younger than 10 years or when comorbid pulmonary, neurologic, or hemodynamically significant structural heart disease coexist.5 Deeper sedation with general anesthesia as well as “breath holding” apnea techniques may be preferred if a para-Hisian septal pathway is suspected to minimize catheter instability related to respiratory motion.5 When no anesthesiologist is present, a nurse with sedation experience is crucial for close monitoring of the airway and vital signs and to avoid sedation overdose, especially during long procedures.
Ablation Energy Source
In the current era, RF energy or cryothermal (cryo) energy is used to interrupt abnormal pathways and ablate ectopic foci. The developing myocardium has the potential for the growth of lesions over time after application of RF energy, but no long-term follow-up studies are available to understand the implications. This potential should be considered when RF ablation is used in young children. Cryo energy application is preferred if the target is near the sinoatrial (SA) node, AV node, or His bundle to prevent damage to these areas of specialized conduction tissue that could result in SA node dysfunction or permanent heart block. Cryo ablation is preferred for ablation inside the coronary sinus as RF energy delivery in this region can result in blood coagulation, inadequate energy delivery, coronary artery injury, or perforation. Long-term recurrence risk of arrhythmia after cryo ablation, which may be as high as 10% to 20%, can be diminished by accurate mapping and requiring stringent time to success of less than 15 seconds after the onset of a cryo lesion before proceeding with a complete lesion.7
Approach to Ablation of Accessory Pathways
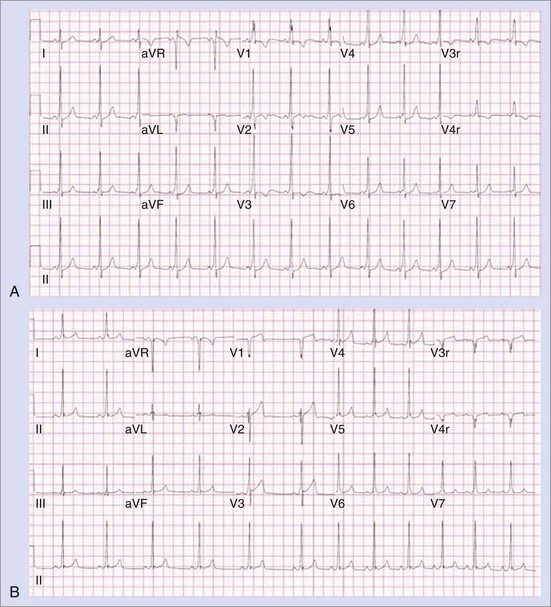
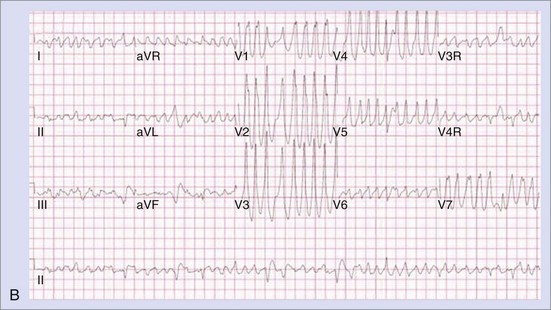
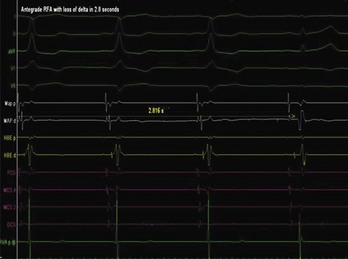
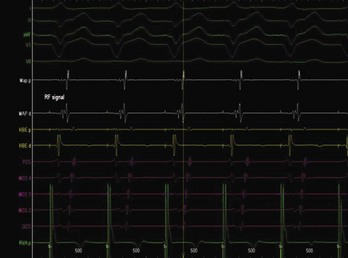
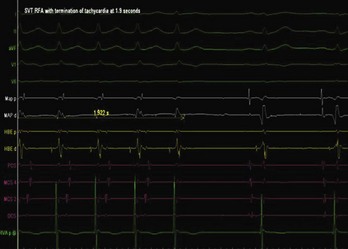
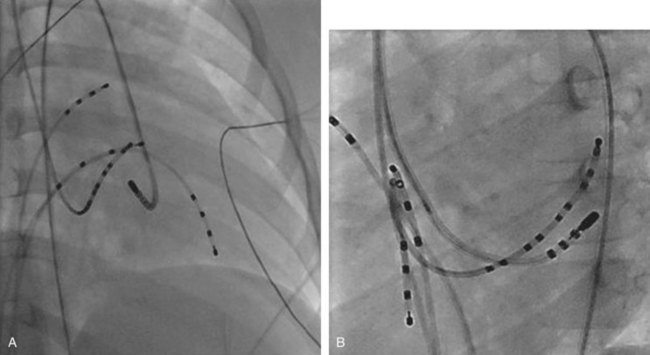
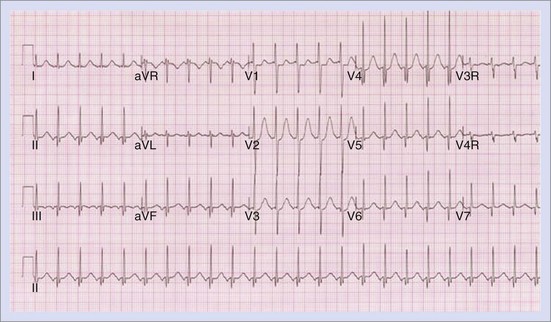
The approach to the ablation of APs depends on whether the pathway is concealed or manifest (in WPW syndrome) (Figure 76-2). Subjects with WPW syndrome should have the antegrade ERP and the shortest pre-excited R-R interval in AF determined. Indicators of high-risk AP include an ERP of less than 250 ms (Figure 76-3, A), the shortest pre-excited R-R interval of less than 220 ms, or the presence of multiple pathways.8–10 More recent studies have reported on a follow-up of 184 asymptomatic patients with WPW syndrome over 57 months (mean age, 10 years); 19 subjects (almost 10%) developed life-threatening arrhythmias, which makes a case for risk stratifying all patients with WPW syndrome at some point during the clinical course of their disease (Figure 76-3, B).11 Mapping of APs can be performed either during sinus rhythm with overt pre-excitation (Figure 76-4), during ventricular pacing by observing retrograde atrial conduction (Figure 76-5), or by observing retrograde atrial activation during SVT (Figure 76-6). If retrograde AP conduction is mapped during ventricular pacing, it must be ensured that fusion with retrograde AV nodal–His-Purkinje conduction, which is quite robust in children, does not occur.12 This can be confirmed with the administration of a bolus of IV adenosine during ventricular pacing, which blocks the retrograde AV nodal conduction but not the retrograde AP conduction. Additionally, other pacing maneuvers such as ventricular PES with single extrastimuli will eliminate retrograde AV nodal conduction. Rarely, children can have adenosine-sensitive antegrade or retrograde conducting APs.
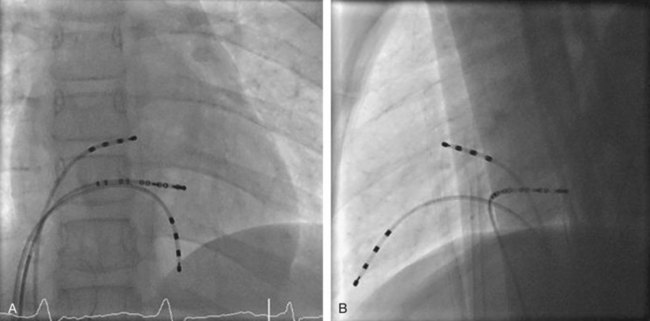
Approximately 60% of all APs are located on the left AV groove.12 Access to the left atrium is achieved by the trans-septal technique using a trans-septal puncture kit (which includes the sheath, the dilator, and the needle) with the assistance of bi-plane fluoroscopy, pressure monitoring, and contrast injection (Figure 76-7). Rarely, transesophageal echocardiogram (TEE) or intracardiac echocardiogram (ICE) may be helpful in the presence of complex heart disease. Heparinization is essential for ablation of left-sided pathways once trans-septal access is obtained; activated clotting time should be maintained above 250 seconds with heparin infusion or bolus doses for the duration of the procedure. In some pediatric laboratories, a retrograde trans-aortic technique is preferred for left-sided pathways (Figure 76-8) with established safety13; the presence of aortic valve disease or younger age (<5 years) may be relative contraindications to this approach because of the risk of damage to the aortic valve from the maneuvering of the ablation catheter.
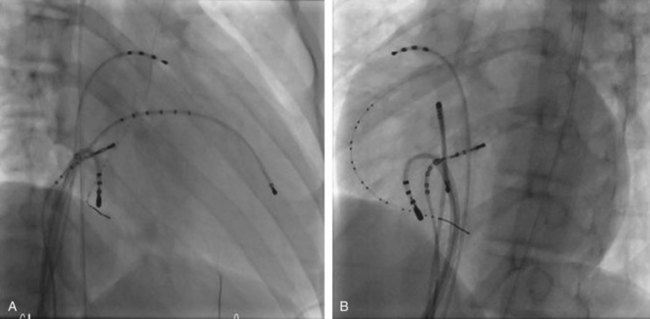
In cases with a left-sided AP, mapping is improved by placing a multiple-electrode catheter in the coronary sinus for initial pathway localization. Access to right-sided pathways, although straightforward from an access standpoint, may be technically challenging. The lower success rate of right AP ablation is related to mapping error and poor catheter stabilization. The tricuspid valve (TV) has a larger circumference than the mitral valve and differs with respect to the angle at which the valve attaches to the annulus. In addition, the absence of a reference catheter in an annular venous structure (such as the coronary sinus) increases mapping errors. The right coronary artery (RCA) runs along the ventricular aspect of the right epicardial AV groove and has a constant anatomic relation to the right AV annulus, regardless of the location of valve attachment. RCA mapping with various catheters has been used for pathway localization in selected patients in some EP laboratories (Figure 76-9). The efficacy and safety of this technique using a multiple-polar 2F microcatheter in a select group of children has been reported.14 Despite accurate mapping of the right AV annulus, the procedure is often confounded by catheter instability. It is often necessary to use a long sheath with specially directed curves that orient the catheter tip toward the right AV groove. Additionally, cryo ablation, by virtue of catheter adherence to the AV groove during a lesion, may be advantageous. This may be specifically helpful in right-sided anteroseptal and midseptal APs. In these locations, accurate mapping with minimal catheter movement is important to avoid any injury to collateral structures such as the His bundle (Figures 76-10 and 76-11). Catheter mapping from the right internal jugular approach is sometimes more helpful than the traditional femoral approach because of the angle of approach to the AV groove.
Approximately 5% to 10% of the patients may have multiple pathways or multiple SVT mechanisms.9 Post-ablation EP testing includes repeat programmed electrical stimulation to exclude the presence of multiple pathways or confirm the recovery of the recently ablated AP. After a presumed successful ablation, adenosine confirms the absence of antegrade or retrograde AP conduction. Isoproterenol is used to provide adrenergic stimulation during programmed electrical stimulation to indicate lack of inducibility of SVT. This protocol is used during the “waiting period” after ablation to confirm a successful ablation with noninduction of SVT and absence of residual or additional APs. The “waiting period” after the successful lesion may vary from 30 to 60 minutes.
Ablation for Atrioventricular Nodal Re-entry Tachycardia
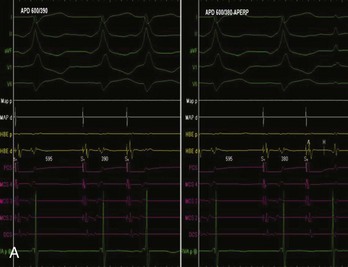
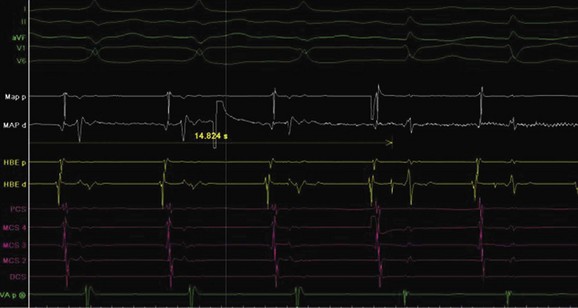
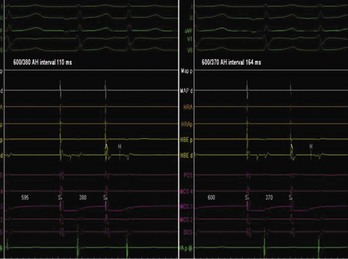
AV nodal re-entry tachycardia (AVNRT) is infrequent in infants and young children, but its incidence increases with age.1 Although the mechanism of AVNRT induction is similar in children and adults, the same definition of dual AV node physiology—that is, 50-ms increase in the atrium to His (A-H) with a 10-ms decrease in atrium-to-atrium (A-A) extrastimulus (Figure 76-12)—may not be applicable to the pediatric population; typical and atypical AVNRT may be induced without clear-cut evidence of dual AV node physiology. Slow pathway elimination or modification is performed by RF or cryo energy delivery to the area of slow conduction in the triangle of Koch. Although the dimensions of the triangle of Koch are relatively constant in adults of different sizes, the area increases with growth in children. In children with a body weight less than 20 kg, the area is 50 mm2 or less.15 This small dimension, along with the proximity of the AV node and His bundle region, should be considered carefully when applying RF energy in small children with AVNRT. With RF application in the slow pathway region, accelerated junctional rhythm is observed. Care needs to be taken to preserve fast pathway conduction (by either atrial pacing or by watching the ventriculoatrial [VA] conduction in junctional rhythm) (Figure 76-13). Successful elimination of AVNRT can be achieved with a 95% success rate and a low recurrence risk by using RF energy. Optimal temperatures during RF ablation should not exceed 55° C and often 45° to 48° C is sufficient.16
Radiofrequency Ablation Versus Cryothermal Ablation
With the advent of cryo technology, more operators are choosing cryo ablation over RF ablation as the first line of approach to avoid AV block. Success and recurrence risks have been reported to be similar in both procedures, but procedure time may be longer in cryo ablation.17 More recent studies have shown overall lower success rate of 83% with cryo ablation with a 6-mm catheter tip in comparison with the standard RF ablation success rate of 93%.7 In addition, this study compared the use of 6-mm cryo catheters for cryo ablation with RF ablation and found a 0.7% incidence of heart block with RF ablation, no heart block with cryo ablation, and no difference in procedure or fluoroscopy times. The use of 8-mm tip cryo catheters has also been shown to be effective, with 91% acute success with a 2.8% long-term recurrence risk18; a 6.5% incidence of transient AV block with complete recovery was seen in all subjects, and pacemaker implantation was not needed in any. Cryo lesions are usually placed in the slow pathway location in a sinus or paced rhythm while monitoring the antegrade fast pathway conduction; junctional rhythm is usually not seen, in contrast to slow pathway modifications with RF energy. Because the cryo ablation catheter is stable, an EPS (using PES) for a slow pathway can be performed during placement of the lesion to assess the efficacy of the cryo ablation.
Ablation for Ectopic Atrial Tachycardia
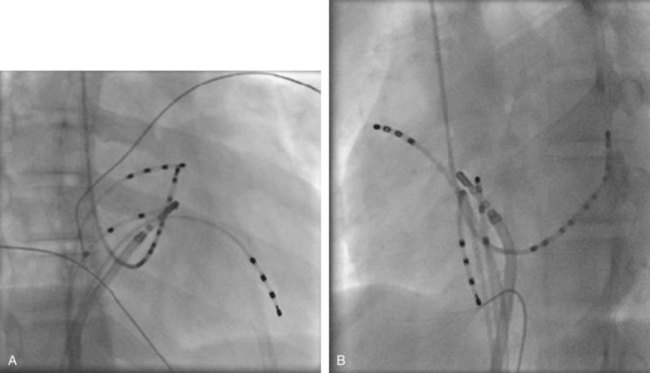
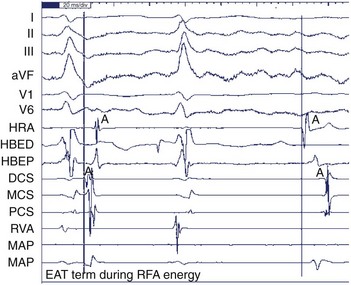
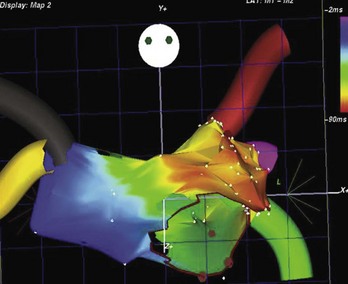
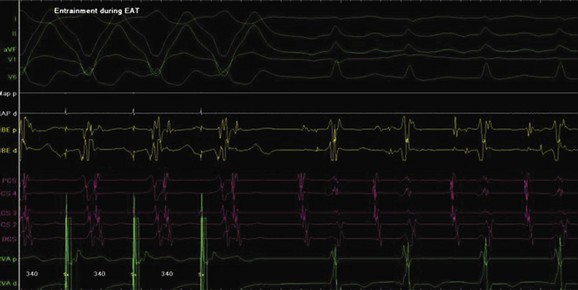
Ectopic atrial tachycardia (EAT) is an uncommon arrhythmia accounting for 5% to 20% of all SVTs.19 The tachycardia may be incessant and result in tachycardia-induced cardiomyopathy. In younger patients (<1 year), conventional drug therapy is attempted, but in older patients, catheter ablation is often preferred, especially if the tachycardia is incessant. A small percentage of patients may have spontaneous resolution of their tachycardia over time.12 PES is often of little help, as the SVT is often focal and automatic; however, it may respond to catecholamines. EATs can be left sided or right sided in origin (Figures 76-14 and 76-15) and sometimes can have a triggered mechanism. Use of conventional mapping with complementary three-dimensional systems, such as contact and noncontact mapping, is often helpful in decreasing fluoroscopy and procedure times and in increasing chances of ablation success.20 Depending on the cycle length and location of the EAT, mapping (Figures 76-16 and 76-17) and entrainment maneuvers (Figure 76-18) are performed before using RF ablation or cryo ablation. In the pediatric population, some of these ectopic tachycardias may be sensitive to sedation and anesthesia and may be noninducible because of suppression of the automatic focus by the sedation or the anesthetic. This may make mapping difficult in the very young, in whom conscious sedation may not be an option. Multiple P-wave morphologies and multiple foci may be present, which necessitates careful mapping in both atria before ablation is performed. Some of the secondary ectopic foci will become apparent after ablation of the initial clinical tachycardia.
Junctional Ectopic Tachycardia
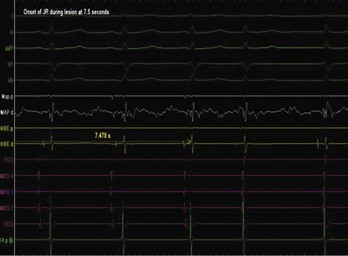
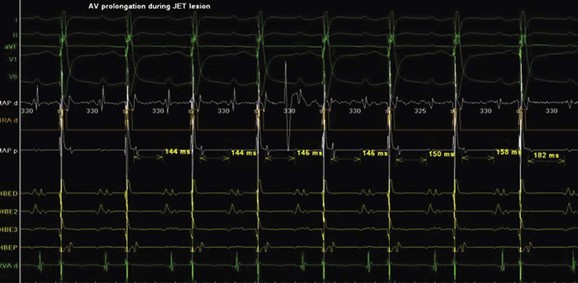
Junctional ectopic tachycardia (JET) is an automatic tachycardia arising from the AV node or the His bundle, which can be seen either as a congenital (Figure 76-19) or as a postoperative form. Congenital JET may present early in life (in the first year of life, shortly after birth) and is often familial in 50% of the cases, and the etiology is unclear.21 Some pathology reports have shown inflammatory changes at the crest of the ventricular septum, and others have linked it to anti-SSA and anti-SSB antibodies.22,23 In contrast, the postoperative form is seen in the immediate postoperative period after repair of ventricular septal defect (VSD) or of complex CHD such as palliation of tetralogy of Fallot, AV canal, and single ventricle and transposition of the great artery.19 Treatment of congenital JET initially usually consists of medical therapy with amiodarone in combination with digoxin, β-blockers, or flecanide. If the tachycardia is incessant with associated hemodynamic compromise, cryo or RF ablation is an option. Because RF has a high risk of causing AV block, cryo ablation is the preferred choice. Mapping is performed during tachycardia, and the region of interest is the anterior septum near the bundle of His. Either RF or cryo energy may be used to successfully eliminate the automatic focus.24,25 The earliest His signal during tachycardia is targeted (Figure 76-20), and care is taken to monitor antegrade conduction and AV or P-R interval during lesion application. Postoperative JET almost never needs invasive intervention and can be managed with medications such as IV amiodarone.19 It is self-limiting a few days after surgery once the inotropic medications are weaned and surgical stresses have abated.
Permanent Junctional Reciprocating Tachycardia
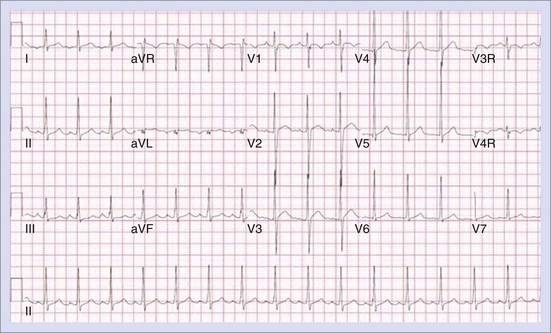
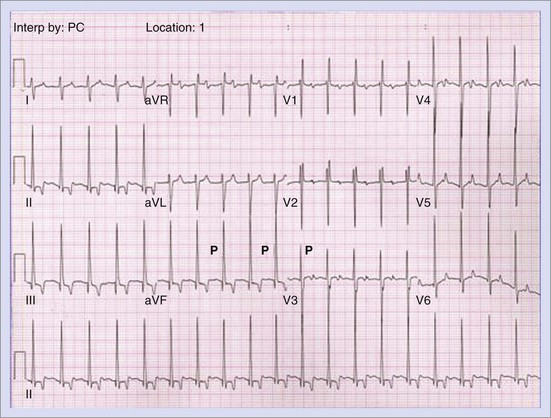
Permanent junctional reciprocating tachycardia (PJRT) is, in fact, a misnomer, as this disease is characterized by an orthodromic reciprocating tachycardia mediated by a slowly conducting concealed accessory pathway.26 Because of the retrograde decremental pathway properties, the tachycardia may be incessant and result in tachycardia-induced cardiomyopathy. The tachycardia is characterized by narrow QRS morphology with a long R-P interval and negative P waves in leads 2 and 3 and aVF with earliest retrograde atrial activation in the posteroseptal right atrium (Figures 76-21 and 76-22). Medical management can be frustrating because of the incessant nature of the tachycardia, so catheter ablation is often the treatment of choice. Differential diagnosis includes atypical AV node re-entry tachycardia and ectopic atrial tachycardia, both of which can be associated with long R-P interval tachycardias. Diagnosis is confirmed by an EPS and maneuvers that include demonstrating atrial pre-excitation with a ventricular premature beat during His refractoriness, response to adenosine, and entrainment with ventricular pacing during tachycardia. Most of these pathways are located in the right posteroseptal region (Figure 76-23), but some have been reported in other locations such as right-lateral and left-posteroseptal AV groove.27 Success rates of RF and cryo ablation may be as high as 95%, with a recurrence risk greater than those with other APs.28 Complications include risk of injury to the AV node and the RCA, especially when ablating in the mouth of the coronary sinus.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree