Unstable Angina: Ischemic Syndromes
Harvey D. White
Overview
The prevalence of non–ST-elevation acute coronary syndromes (NSTEACS) is increasing. The major pathophysiologic mechanism is plaque rupture or fissuring with superimposed thrombus. Risk profiling should be performed at admission and repeated on several subsequent occasions to incorporate new information regarding the patient’s response to therapy and risk factors. Treatment should be tailored according to individual patient characteristics and risk.
Patients should initially receive intensive antithrombotic therapy to passivate the thrombotic activity of the culprit unstable plaque. The optimal antithrombotic regimen has not yet been defined. Aspirin reduces the risk of cardiac events by 20%. In comparison with unfractionated heparin (UFH), enoxaparin has been shown to reduce the combined incidence of death and myocardial infarction (MI) by 9% at 30 days, and may be favored for patients being managed conservatively because of its ease of use. In patients managed invasively, enoxaparin and UFH produce similar outcomes, although enoxaparin is associated with a modest increase in bleeding. In patients not selected for early percutaneous coronary intervention (PCI), glycoprotein (GP) IIb/IIIa antagonists have been shown to reduce 30-day death/MI rates by 9% overall and by 18% in patients with elevated troponin levels, although there was no benefit in patients
without elevated troponin levels. Adjunctive use of clopidogrel with aspirin has a synergistic effect, reducing cardiovascular death/MI/stroke by 20% in patients with and patients without elevated troponin levels.
without elevated troponin levels. Adjunctive use of clopidogrel with aspirin has a synergistic effect, reducing cardiovascular death/MI/stroke by 20% in patients with and patients without elevated troponin levels.
β-Blockers, nitrates, and calcium channel antagonists relieve angina, but have no significant effect on intracoronary thrombus and do not necessarily reduce the risk of death/MI. The risk of cardiac events is decreased by reduction of low-density lipoprotein cholesterol (LDL-C) levels to below 62 mg/dL (1.6 mmol/L) using early aggressive statin therapy.
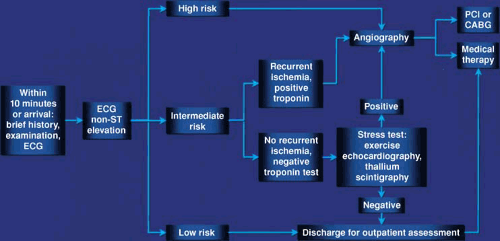
Early angiography and revascularization are integral to the management of NSTEACS. When compared with conservative medical treatment, early revascularization reduces the risk of death/MI in high-risk patients, decreases the need for antianginal medications, allows a shorter hospital stay, and results in fewer readmissions. This approach is cost effective in many healthcare settings. Patients selected for early revascularization should have the procedure within 48 hours of admission; the optimal timing of intervention has not yet been established. Intermediate-risk patients should be admitted to a coronary care or chest pain unit, monitored closely, and given intensive antithrombotic therapy. If patients become high-risk (i.e., they have recent ischemia or their troponin levels rise), they should have early angiography and revascularization if appropriate. If symptoms settle and troponin levels are not elevated, patients should be managed according to whether or not they have inducible ischemia. Low-risk patients should be discharged early with appropriate arrangements for follow-up and review.
The risk of ischemic events remains high in patients with NSTEACS, and management continues to pose a major clinical challenge. Better treatments and new therapeutic strategies are needed. It is important that evidence-based therapies be used and that primary and secondary preventative measures be instituted to reduce the community burden of acute coronary syndromes (ACS).
Glossary
Conservative management
Risk profiling and medical therapy with selective use of angiography and revascularization procedures depending on symptoms, response to therapy, and the presence of inducible ischemia at low workloads or low doses of pharmacologic agents.
Invasive management
Medical therapy plus early coronary angiography and revascularization.
Plaque instability
Propensity for atheromatous plaque to rupture or fissure.
Plaque passivation
Inactivation of the platelet-active surface of a ruptured or fissured plaque.
Rebound ischemia
Increase in ischemic events when heparin therapy is stopped.
Risk profiling
Estimation of the risk of coronary events (usually death or MI) by assessment of patient characteristics and investigative findings.
Introduction
The first documented description of a patient with an acute ischemic syndrome is in the Ebers papyrus from 2,600 BCE, which states, “If you find a man with heart discomfort, with pain in his arms, at the side of his heart, death is near.” This description remains apt, but the prognosis has changed over the centuries. Today, unstable angina is one of the commonest causes of hospitalization (1). Each year, more than 1.4 million patients in the United States (2) and more than 4 million worldwide (3) are hospitalized with NSTEACS. These numbers will continue to rise as the prevalence of patients with obesity and diabetes increases (3).
The syndromes of unstable angina, non–ST-elevation MI (NSTEMI) and ST-elevation MI (STEMI) are a continuum, and the pathophysiology is heterogeneous and dynamic. Clinical presentation depends on the severity of the arterial injury, the size and type of thrombus formed, the extent and duration of ischemia, and the amount of previous myocardial necrosis. The extent of ischemia depends on the myocardial distribution of the ischemia-producing artery, the severity of the ischemia-producing stenosis, the absence or presence of collateral circulation, and factors that affect the supply of oxygenated blood or that increase myocardial demands, such as the heart rate, blood pressure, and contractility. Patients may die or may develop MI, recurrent ischemia, heart failure, arrhythmia, or a stroke.
Definitions
Acute coronary syndromes describes a spectrum of clinical syndromes ranging from unstable angina to NSTEMI and STEMI. Patients presenting with ACS are divided into those with ST elevation (lasting ≥20 minutes) or new left bundle branch block, and those with NSTEACS which includes transient ST elevation (lasting <20 minutes), unstable angina, and NSTEMI (4).
Unstable angina is a syndrome intermediate between chronic stable angina and MI. It is a clinical diagnosis based on a history of chest pain and exclusion of the diagnosis of MI by electrocardiography (ECG) and biomarker testing for myocardial necrosis. The chest pain may be prolonged at rest or of new onset, may represent accelerating symptoms of previously stable angina, or may occur after MI. Patients presenting without ST elevation on the ECG are diagnosed as having either NSTEMI or unstable angina based on whether or not their troponin or creatine kinase (CK)-MB levels are elevated (5). Between 2% and 15% of patients diagnosed with unstable angina subsequently develop Q-wave MI.
The unstable angina classification developed by Braunwald (6) is based on the severity of symptoms, their clinical context, and the intensity of medical treatment. The classification has been validated clinically (7), has been shown to correlate with coronary angiographic findings (8), and has now been updated to include troponin levels (Table 18.1) (9). Prinzmetal angina (recurrent rest angina accompanied by ST elevation on the ECG owing to coronary artery spasm) is considered a separate entity (10).
Pathophysiology
The five major causes of ACS are thrombus, mechanical obstruction, dynamic obstruction, inflammation, and increased oxygen demand (11). The major pathophysiologic mechanism is rupture or fissuring of an atheromatous plaque with superimposed thrombus (12,13,14,15). Other mechanisms include superficial erosion (which is more common in women), intraplaque hemorrhage, and erosion of a calcified nodule (16). Patients with ACS often have more than one ulcerated plaque, as shown by angiography (17), intravascular ultrasound (18), angioscopy (19), and release of inflammatory markers such as myeloperoxidase across nonculprit coronary vascular beds (20). Multiple plaque ruptures are more common in patients with increased C-reactive protein (CRP) levels (21).
In some patients, thrombogenicity of the blood (sometimes referred to as “vulnerable blood” leading to the concept of “vulnerable patients”) is implicated, with alterations in circulatory prothrombotic or antifibrinolytic mechanisms. Levels of
plasminogen activator inhibitor-1 are increased in patients with obesity or diabetes (22).
plasminogen activator inhibitor-1 are increased in patients with obesity or diabetes (22).
| ||||||||||||||||||||||||
Superficial fissuring of a plaque usually results in platelet deposition. There is less superimposed thrombus formation in patients with NSTEACS than in those with STEMI, which is usually associated with deep arterial injury and occlusive thrombus (Fig. 18.1) (14,15). Angioscopic findings show that the thrombus associated with unstable angina is white or gray and consists mostly of platelets, whereas the thrombus associated with STEMI consists mostly of red blood cells (23).
Inflammation plays a major role in atherosclerosis (24,25), and activation of macrophages triggers inflammatory processes that lead to plaque instability, procoagulation, and clinical events. Plaque rupture or fissuring can be triggered by increased shear forces with sudden changes in pressure or tone. Rupture most often occurs on minor plaques (26,27,28,29,30,31) that are eccentric (32) and have a large lipid core with a thin fibrous cap (33), increased concentrations of macrophages (34), and local expression of tissue factor. Macrophages produce metalloproteases such as collagenase, elastases, and stromelysins, which digest extracellular matrix (25). Macrophage-rich areas are more commonly found in atherectomy specimens from patients with unstable angina than from those with stable angina (35). Activated T lymphocytes are present at sites of plaque rupture (36) and they release various cytokines which activate macrophages and promote smooth muscle cell proliferation (25). Mast cells are found on plaque edges at sites that are likely to rupture (37). Increased levels of CRP (38) and its major inducer, interleukin-6 (39), have been found in patients with unstable angina, and are associated with higher rates of death/MI at hospital discharge and at 1 year (40,41,42).
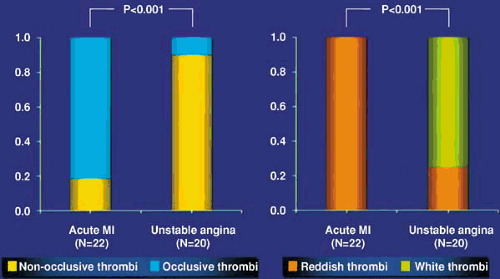 FIGURE 18.1. Angioscopic findings in acute ischemic syndromes showing (left) the proportions of occlusive and nonocclusive thrombi and (right) the differing character of thrombi in acute MI and unstable angina (23). (Source: Redrawn from Mizuno K. Angioscopy in acute coronary syndromes. Cardiology Today 1992;20:1 , with permission.) |
The inflammatory stimulus for triggering expression of soluble cell adhesion molecules has been shown to persist for 6 months after presentation with NSTEACS (43). The inflammatory nature of the cells at the site of plaque rupture and shared T-cell receptor sequences in clonotypes from different patients (43,44) have led to speculation that chronic stimulation by a common antigen or certain bacterial infections, such as Chlamydia pneumoniae or Helicobacter pylori, may be associated with an increased risk of plaque rupture (45,46,47).
The T-cell response in patients with unstable angina is antigen-driven and directed toward antigens carried in culprit coronary atherosclerotic plaques (48). Cytomegalovirus has been found in atheromatous plaque specimens, but active replication of the virus is not thought to be a major cause of plaque instability (49).
The T-cell response in patients with unstable angina is antigen-driven and directed toward antigens carried in culprit coronary atherosclerotic plaques (48). Cytomegalovirus has been found in atheromatous plaque specimens, but active replication of the virus is not thought to be a major cause of plaque instability (49).
After plaque rupture or fissuring, subendothelial adhesive proteins, collagen tissue factor, and von Willebrand factor are exposed, and tissue factor is released. Platelets adhere to GP Ia and Ib, change their shape, and release serotonin, thromboxane A2, and adenosine diphosphate (ADP). In animal models, episodic platelet aggregation at sites of coronary stenosis has been shown to cause cyclic coronary blood flow (50). Platelet emboli have been found downstream from atheromatous plaques in small intramyocardial vessels from patients who have died suddenly (51). Platelet activation may manifest in anginal episodes associated with increased urinary levels of thromboxane B2 (52).
Released tissue factor combines with factor VII, stimulating the extrinsic coagulation cascade to form thrombin, a very potent stimulus of platelet aggregation. At the platelet surface, factors V and X are activated to form the prothrombinase complex, which generates more thrombin. Damage to endothelium without plaque rupture may also result in thrombus formation (53). Evidence of a hypercoagulable state has been found in patients with unstable angina (54). During anginal episodes, increases occur in the plasma concentrations of prothrombin fragments 1 and 2 (signifying increased activity of factor Xa and thrombin formation) and fibrinopeptide A (a sign of increased thrombin activity and fibrin formation). These markers remain elevated for at least 6 months after ACS (55), and platelets remain activated for at least 28 days (56). Intracoronary thrombus is visualized in 35% to 52% of patients having coronary angiography for unstable angina (8,57,58,59); the detection rate rises to 70% to 93% when angioscopy is performed (23,58,60,61,62). The presence of thrombus at angiography denotes an increased risk of recurrent ischemia and MI (57). Another potential mechanism of ACS is increased narrowing of a coronary artery due to progression of atherosclerosis or plaque rupture (63).
Cocaine is toxic to the heart, and its use may be associated with ACS, even in patients with angiographically normal coronary arteries (64). There is a circadian variation in the onset of ACS (65). Platelet aggregation increases in the morning, elevating the risk of MI or sudden death (66). Activities such as heavy exertion, which produces acute physiologic effects, may also trigger ischemic events (67).
Coronary Artery Spasm
In 1959, Prinzmetal et al. (10) described a variant form of angina characterized by chest pain predominantly at rest and usually associated with ST elevation on the ECG. Rarely, variant angina is associated with other vasospastic disorders (such as migraine or Raynaud syndrome) in patients with angiographically normal coronary arteries.
Physiologic contraction of an epicardial coronary artery may be triggered by stimuli such as increased adrenergic activity (68), increased vagal activity (69), secretion of vasoconstrictor substances such as thromboxane A2 (70), and increased production of endothelin-1 by endothelial cells (71). Increased vasomotor hyperreactivity may be localized to regions of coronary atheroma or may occur in angiographically normal arterial segments (72). In the presence of endothelial dysfunction, stimuli such as acetylcholine that normally cause vasodilation may instead cause vasoconstriction (73). In the presence of a severe atherosclerotic stenosis (particularly one that is eccentric with an arc of normal coronary artery that is able to contract), increased coronary tone can cause a critical reduction in coronary blood flow. Cold-pressor testing has demonstrated excessive vasoreactivity in unstable angina patients compared with stable angina patients (74).
Pathophysiologic Implications for Clinical Management
The management of patients with NSTEACS should focus on the pathophysiology (11). The primary aims of treatment are to reduce initial symptoms and ischemia, prevent MI, minimize necrosis in the event of MI, and reduce mortality. In individual patients, the mechanisms of plaque rupture or fissuring, platelet aggregation, thrombus formation, and increased vasomotor tone may play different roles at different times. A variety of therapeutic approaches are needed to modify these processes.
The mainstays of medical management are intensive antithrombotic therapy with aspirin and heparin (75) (either UFH [75] or low-molecular-weight heparin [LMWH] [76]), and antiplatelet therapy with clopidogrel (77) and/or GP IIb/IIIa antagonists (78). β-Blockers, nitrates, and calcium channel antagonists should be used for relief of symptoms. Early angiography and revascularization are recommended for patients at high risk or with recurrent symptoms. Risk profiling is pivotal to triage, and the results determine whether patients should be discharged early, admitted and monitored closely, or have early angiography and revascularization (Fig. 18.2).
Patients with an increased oxygen demand or a decreased oxygen supply (e.g., those with anemia or thyrotoxicosis) need to be managed appropriately. Patients with vasospasm require therapies such as nitrates and calcium channel antagonists. It has not yet been established how patients with evidence of inflammation should be managed, although falls in CRP levels have been noted with aspirin (79) and with statins, which improve outcomes independently of their effect on LDL-C (80,81).
Clinical Profile
History and Physical Examination
The physical findings and the site, character, and radiation of the discomfort are similar to those seen in patients with MI. The physical examination is usually normal unless ischemia causes signs of poor tissue perfusion, with sweating, tachycardia, cool extremities, third or fourth heart sounds, and signs of heart failure or cardiogenic shock.
Electrocardiography
The ECG is a very important investigative tool; prognosis and management critical depend on ECG findings. An ECG should be performed at admission, daily throughout hospitalization, and during episodes of ischemia. If there are symptoms lasting longer than 20 minutes with ST elevation or new left bundle branch block, administration of fibrinolytic therapy or primary PCI should be considered. A normal ECG does not exclude the possibility of an ACS. Transient ST depression (or, less frequently, elevation) and T-wave inversion occur commonly only during ischemia (82,83,84,85).
In the Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO)-IIB trial, patients with 2 mm of ST depression in two ECG leads had 6 to 10 times the mortality rate of patients with normal ECGs (86). ST depression of 0.5 mm or more has been shown to be a significant risk factor for
death/MI at 1 (84) and 4 years (85). In GUSTO-IIB, patients with ST depression were more likely to have triple-vessel disease (36%), whereas those with T-wave inversion were more likely to have normal coronary arteries (19%). Thirty-day mortality was 5.1% in those with ST depression and 1.7% in those with isolated T-wave inversion (83). Patients with inverted T waves in 5 leads or more are at higher risk (87) and have better outcomes with revascularization than with conservative therapy (88). Little information is available regarding the outcome of patients with deep T-wave inversion (>0.2 mV), who are usually classified as being at intermediate risk.
death/MI at 1 (84) and 4 years (85). In GUSTO-IIB, patients with ST depression were more likely to have triple-vessel disease (36%), whereas those with T-wave inversion were more likely to have normal coronary arteries (19%). Thirty-day mortality was 5.1% in those with ST depression and 1.7% in those with isolated T-wave inversion (83). Patients with inverted T waves in 5 leads or more are at higher risk (87) and have better outcomes with revascularization than with conservative therapy (88). Little information is available regarding the outcome of patients with deep T-wave inversion (>0.2 mV), who are usually classified as being at intermediate risk.
In the Fragmin During Instability in Coronary Artery Disease (FRISC) trial, the benefit of early revascularization was proportional to the depth of ST depression (89), and this association was independent of age, gender, and troponin levels. Revascularization was most beneficial in patients who had both ST depression and elevated troponin levels (90). However, the Treat Angina With Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy–Thrombolysis in Myocardial Infarction-18 (TACTICS–TIMI-18) trial (91) and the Randomised Intervention Trial of Unstable Angina (RITA-3) trial (92) found no association between ST-segment changes and the benefits of revascularization.
Continuous Electrocardiographic Monitoring
Ischemic ST-segment changes are detected on continuous ECG monitoring in 85% to 90% of patients with unstable angina, but the changes are often silent (93,94). Silent ischemia during Holter monitoring has been shown to correlate with reduced myocardial perfusion and impaired ventricular function (94), and patients with silent ischemia are more likely to die, develop MI, or require revascularization (94,95,96). The European Society of Cardiology (ESC) guidelines for the management of NSTEACS (97) recommend that patients should have multilead continuous ST-segment monitoring if it is available or, failing that, frequent ECGs.
Chest X-Ray
Unless MI has occurred previously, heart size is usually normal. Transient pulmonary edema may occur with global ischemia, and suggests the possibility of a left main coronary stenosis.
Troponins
The cardiac troponins are sensitive and specific markers of myocyte necrosis (98) and are the markers of choice for the diagnosis of MI (5). Short- and long-term studies have shown that troponin levels correlate with the risk of death and the combined risk of death/MI (99,100,101,102,103), with a clear gradient of risk as troponin levels increase (100,104). Troponin levels have been shown to be more powerful prognosticators than CK-MB levels (105). Thirty percent of patients who present with NSTEACS and normal CK-MB levels have elevated troponin levels (100,102,106), and these patients have poor outcomes. The combination of troponin T testing and exercise testing further defines patients at low, intermediate, and high risk (107).
Elevated troponin levels correlate with the pathophysiology of ACS (the presence of thrombus in the coronary artery) (108), and reflect the thrombogenic activity of ruptured or fissured plaques with embolism downstream and resultant myocyte necrosis (90,108). The prognostic value of troponins is greater than would be expected from the extent of myocyte necrosis and left ventricular impairment, perhaps reflecting preceding episodes of embolic episodes (Fig. 18.3) (109). Angiographic studies have shown that evidence of thrombus, complex lesions, and a reduced TIMI flow grade (110) were more common in patients with elevated troponin levels than in those with normal levels (90,108).
Troponin levels identify patients who are most likely to benefit from LMWH (106), GP IIb/IIIa antagonists (104,111,112), and revascularization (91,113). Troponin testing may be the only biomarker assay needed if utilized in a chest pain pathway (114). Point-of-care testing is recommended in institutions that cannot consistently deliver laboratory results within 1 hour for logistical reasons (115). Baseline point-of-care use of a multimarker assay including myoglobin (which is released earlier than troponins) has been shown to be a more effective means of risk profiling than single-marker, laboratory-based testing (116).
Troponins are very sensitive markers of myocyte necrosis, and elevated levels may be detected in contexts other than spontaneous myocardial ischemia or PCI (117). Apart from ACS, the most common causes of elevated troponin levels are atrial or ventricular tachycardia (often with hypotension and an increased myocardial oxygen demand), pulmonary emboli with right ventricular MI, cardiac failure (118), cardiac surgery,
myocarditis, and renal failure. Other tests such as myosin light-chain assays (119) are not currently recommended as standard practice.
myocarditis, and renal failure. Other tests such as myosin light-chain assays (119) are not currently recommended as standard practice.
 FIGURE 18.3. Microvascular obstruction after plaque rupture (109). (Source: Adapted from Goldmann BU, Christenson RH, Hamm CW, et al. Implications of troponin testing in clinical medicine. Curr Control Trials Cardiovasc Med 2001;2:75–84 , with permission.) |
White Blood Cell Count
Renal Function
Inflammatory Markers
There has been extensive research into the roles of inflammation and inflammatory markers in NSTEACS. The levels of high-sensitivity CRP, interleukin-6 and, more recently, CD-40 ligand (which has prothrombotic effects) have been shown to provide independent prognostic information (128). Elevated levels of other inflammatory markers such as adhesion molecules (129), interleukin-7 (130), and matrix-metalloproteinases (including pregnancy-associated plasma protein A) (131) have also been observed in patients with NSTEACS.
C-Reactive Protein
CRP is an acute-phase protein produced by the liver when there is tissue injury, infection, or inflammation. High-sensitivity CRP levels are elevated in 50% to 70% of patients with Braunwald class IIIB angina (40). Patients with elevated CRP levels at admission have been shown to have worse in-hospital and 1-year outcomes, and elevated levels at discharge have been associated with recurrent instability in the long term (41). In the TIMI-11A study, mortality at 14 days was 9.1% when both CRP and troponin levels were elevated compared with 4.7% if either was elevated and 0.4% if neither was elevated (132). The major application of CRP testing appears to be in determining the long-term prognosis after hospital discharge. Low CRP levels have been observed with aspirin (79) and statin therapy (133).
CD40 Ligand
Amyloid A
Amyloid A is an acute-phase protein produced by the liver. Its predictive value appears to be similar to that of CRP (40).
Fibrinopeptide A
Fibrinopeptide A is a polypeptide cleaved from fibrinogen by thrombin. It is a sensitive marker of thrombin activity and fibrin generation. Elevated urinary fibrinopeptide A levels are associated with the presence of intracoronary thrombus (138) and signify an increased risk of death, MI, or revascularization. Persistently elevated levels denote an increased risk of coronary events (55).
B-Type Natriuretic Peptide
B-type natriuretic peptide (BNP) and the N-terminal fragment of the BNP prohormone (NT-proBNP) are synthesized in the
ventricles, and are important markers of neurohormonal activity. BNP levels correlate with left ventricular pressure, and increase in response to myocardial stretching in the event of myocardial ischemia (139). Several studies have shown that BNP and NT-proBNP levels have powerful prognostic value for death and MI in patients with NSTEACS (140,141,142,143), independent of markers of myocardial necrosis or inflammation. The FRISC-II trial (144) found that BNP levels predicted the benefit of revascularization, but there was no such association in TACTICS–TIMI-18 (142,145). Serial BNP measurements can be used for dynamic risk profiling (Fig. 18.4) (141). Patients with normal troponin levels and low BNP levels are at very low risk of cardiovascular events (Fig. 18.5) (141).
ventricles, and are important markers of neurohormonal activity. BNP levels correlate with left ventricular pressure, and increase in response to myocardial stretching in the event of myocardial ischemia (139). Several studies have shown that BNP and NT-proBNP levels have powerful prognostic value for death and MI in patients with NSTEACS (140,141,142,143), independent of markers of myocardial necrosis or inflammation. The FRISC-II trial (144) found that BNP levels predicted the benefit of revascularization, but there was no such association in TACTICS–TIMI-18 (142,145). Serial BNP measurements can be used for dynamic risk profiling (Fig. 18.4) (141). Patients with normal troponin levels and low BNP levels are at very low risk of cardiovascular events (Fig. 18.5) (141).
 FIGURE 18.4. Predictive value of baseline NT-proBNP in relation to presence and absence of myocardial necrosis as evidenced by elevated levels of TnT (n = 1791) (141). *P < .01 versus NT-proBNP ≤250 ng/L. (Source: Redrawn from Heeschen C, Hamm CW, Mitrovic V, et al. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation 2004;110:3206–3212 , with permission.) |
Other Markers
A number of other markers are currently under investigation, including interleukin-6, intercellular adhesion molecule-1, lipoprotein-associated phospholipase A2, and various tissue
inhibitors of metalloproteinases. Current guidelines do not recommend routine assessment of inflammatory markers.
inhibitors of metalloproteinases. Current guidelines do not recommend routine assessment of inflammatory markers.
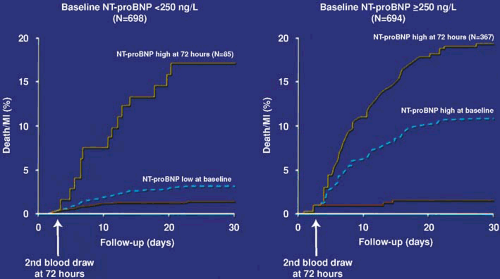 FIGURE 18.5. Dynamic risk profiling in patients with ACS using serial NT-proBNP measurements (n = 1392 patients without death/MI during the first 72 hours) (141). Despite NT-proBNP levels of <250 ng/L at baseline, an increase in NT-proBNP levels during the following 72 hours indicated an adverse clinical course for these patients (left). In contrast, in patients with NT-proBNP levels >250 ng/L at baseline, rapid decline over the following 72 hours indicated low cardiac risk during the subsequent 27 days, whereas patients with consistently high NT-proBNP levels continued to be at increased cardiac risk (right). The dashed lines indicate event rate curves based on baseline NT-proBNP levels. (Source: Redrawn from Heeschen C, Hamm CW, Mitrovic V, et al. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation 2004;110:3206–3212 , with permission.) |
Other Laboratory Tests
Primary risk factors, such as cholesterol and glucose levels, should be assessed at admission. Possible secondary causes of unstable angina should be investigated depending on the clinical circumstances, namely anemia, thyrotoxicosis, pulmonary embolism, and aortic dissection.
Risk Profiling
Risk profiling is critical because it determines the choice of treatment strategy and provides prognostic information for the patient and relatives. It also enhances the cost effectiveness of patient care by allowing evidence-based treatments to be targeted at patients most likely to benefit from them. Risk profiling should take into account clinical factors, ECG features, cardiac marker levels, evidence of spontaneous or inducible ischemia, measures of left ventricular function, and coronary anatomy (Table 18.2) (1,146,147). Certain clinical features are not included in risk profiling models, and the clinical assessment should always be regarded as paramount. For instance, a patient who is gray, sweating, and anxious is likely to be at higher risk than one who is relaxed and appears well. Patients should be assessed fully at presentation and then reviewed at 6 to 8 hours for recurrence of ischemia, response to treatment, and the results of cardiac marker tests, particularly the troponins. Further risk profiling should be done at 12 and 24 hours and again before discharge.
| ||||||||||||||||||||||||||||
Low-risk patients should be discharged early, advised to report any changes in symptoms (e.g., recurring discomfort at rest or at night), and reviewed subsequently at an outpatient clinic. Intermediate-risk patients should receive intensive antithrombotic therapy and close monitoring. If recurrent ischemia occurs or troponin levels rise, early angiography should be performed with a view to revascularization. If symptoms settle and troponin levels do not rise, tests for inducible ischemia should be performed. If the tests show ischemia at a low workload, angiography should be performed with revascularization as appropriate; otherwise, the patient can be managed medically.
High-risk patients should receive intensive antithrombotic therapy and have early angiography and revascularization if their coronary anatomy is suitable.
High-risk patients should receive intensive antithrombotic therapy and have early angiography and revascularization if their coronary anatomy is suitable.
TABLE 18.3 Factors Included in Risk Profiling Models for Patients with Nsteacs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Various risk models have been developed for NSTEACS (Table 18.3) (148,149,150,151,152). The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) investigators (149) found that most of the prognostic information was provided by seven variables: age, gender, Canadian Cardiovascular Society angina classification, heart rate, blood pressure, presence of rales, and presence of ST depression. Different models were developed for the endpoints of death and death/MI, and for NSTEMI and NSTEACS without MI.
The TIMI-11B risk model is based on seven readily quantifiable variables: age 65 years or older, three or more risk factors for coronary artery disease (CAD), prior coronary stenosis of 50% or more, ST-segment changes at presentation, two or more anginal events within the previous 24 hours, use of aspirin within the previous 7 days, and elevated serum cardiac marker levels (150). The advantage of this model is its simplicity. An electronic version of the model is available for palmtop computers (153).
The FRISC risk score includes seven risk factors: age over 70 years, male gender, diabetes, previous MI, ST depression, increased troponin levels, and inflammatory markers (CRP or interleukin-6) (152). In FRISC-II (113), invasive treatment was most beneficial in patients with five or more of these factors who had a 12-month relative risk (RR) for death/MI of 0.34 (95% confidence interval [CI] 0.15–0.78) versus 0.69 (95% CI 0.50–0.94) in patients with three or four risk factors (152). Invasive treatment had no benefit in patients with two or more risk factors. The Global Registry of Acute Coronary Events (GRACE) risk algorithm (151) was developed from an international registry. It includes renal function, and may perform slightly better than trial-based risk scores (154,155).
Noninvasive Investigations
Stress Testing
Exercise or pharmacologic testing (with or without imaging) can be performed as part of a chest pain unit assessment or when patients have been asymptomatic on antithrombotic therapy for 24 to 48 hours. Ischemia occurring at a low workload (<6 metabolic equivalents) is associated with a poor prognosis (156). When exercise testing for ischemia is negative in patients with a normal baseline ECG, the 5-year survival rate is 95% (156). Exercise thallium imaging can be used to assess the severity of CAD and the risk of subsequent cardiac events in patients with unstable angina (157,158).
Some patients have physical limitations that preclude exercise testing and others have ECG changes that are difficult to interpret because of baseline abnormalities such as left ventricular hypertrophy, left bundle branch block, preexcitation, or the effects of digoxin. Pharmacologic stress testing is of particular value in these patients (159). Although stress testing can indicate the presence of severe coronary stenoses (160) and the likelihood of multivessel disease, it cannot detect instability of coronary artery plaques.
Echocardiography
Two-dimensional echocardiography can provide anatomic and functional information, which is helpful in determining the diagnosis and prognosis. Transient wall motion abnormalities and changes in ventricular volumes can be detected during ischemia (161). These findings may be helpful if symptoms are atypical or if ECG findings are nondiagnostic. Echocardiographic changes may precede chest pain or ischemic ST-segment changes. Transesophageal echocardiography is particularly
valuable for evaluating the possibility of aortic dissection and structural abnormalities of the mitral valve. Dobutamine stress echocardiography is very useful for assessing ischemia and the viability of myocardium.
valuable for evaluating the possibility of aortic dissection and structural abnormalities of the mitral valve. Dobutamine stress echocardiography is very useful for assessing ischemia and the viability of myocardium.
Cardiac Magnetic Resonance Imaging
Coronary Angiography
The findings at coronary angiography depend on the population studied. Angiography outlines only the arterial lumen, and may not detect large plaques within the arterial wall (163). In the TIMI-IIIB trial (166), 19% of patients had no coronary stenoses of more than 60% narrowing, and 4% had a left main coronary stenosis of more than 50%. Single-vessel disease was found in 38%, double-vessel disease in 29%, and triple-vessel disease in 15%. Eccentric plaques and complex plaques (Fig. 18.6) are more common in patients with unstable angina than in those with chronic stable angina (8). Coronary artery thrombi may be detected in 40% of patients having angiography soon after an episode of rest pain (57). Impaired coronary flow is common (167,168).
Left Ventriculography
Left ventriculography may detect abnormalities of regional wall motion caused by previous MI or “hibernation” owing to prolonged or recurrent ischemia. Wall motion abnormalities and changes in ventricular volumes may occur during episodes of acute ischemia (169). The presence of mitral regurgitation can be detected and its severity assessed.
Prognosis
The prognosis is worse in patients with NSTEACS than in patients with STEMI (83). Within 1 month, 2% to 5% die and 5% to 16% have an MI (91,170,171). Within 1 year, 26% to 35% require readmission to hospital for recurrent symptoms (172) and 4% to 15% die (166,172,173,174,175,176). Patients with unstable angina and a normal coronary angiogram have good short- (177) and long-term prognoses (178).
In a TACTICS–TIMI-18 substudy of patients without stenoses of 50% or more (179), the 6-month death/MI rate was 3.1% in those with elevated troponin I levels versus 0% in those without elevated troponin I levels. These patients may have had elevated troponin levels for another reason, or they may have had coronary atherothrombosis undetected by angiography. Coronary artery spasm may also have played a role (117).
Clinical Variables
The most important prognostic variables are age (149




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree