Chronic Stable Coronary Disease
Keith A. A. Fox
Introduction
The prevalence of occult atheromatous coronary disease among industrialized communities is so high that the incidental finding of coronary disease on pathologic examination is the norm rather than the exception (see Atherosclerotic Biology and Epidemiology of Disease). Thus, most individuals with nonobstructive coronary arterial disease are asymptomatic and manifest no clinical signs nor symptoms of the disease process. Managing the preclinical phase of the disease, including risk factors modification is considered in Preventive Cardiology. This chapter focuses on chronic stable coronary disease manifest through symptoms, clinical signs, or cardiac complications.
Chronic stable angina is the most common symptomatic manifestation of obstructive coronary artery disease. Although myocardial oxygen supply–demand imbalance may result in angina in the absence of detectable atheromatous coronary artery disease, the vast majority of angina occurs in the presence of obstructive coronary artery plaques. However, the threshold for provoking angina and the severity of symptoms depend on a variety of factors that influence loading conditions, oxygen demand, and cellular cytoprotective pathways because myocardial ischemia is more prevalent than symptomatic angina and the threshold for provoking symptoms varies within and between patients. Rather than an isolated condition, chronic stable angina should be regarded as a symptomatic manifestation of predominantly obstructive coronary artery disease and a relatively stable interlude in the pathophysiology of progression of atheromatous coronary artery disease.
Despite the absence of symptoms and clinical manifestations early in the progression of coronary disease, markers of the disease process “intermediate phenotypes” may be detectable biochemically and by noninvasive and invasive testing and prognosis may be altered by interventions that aim to mitigate the progression of atheroma.
Prevalence and Incidence of Angina
The elegant description by William Heberden in 1768 captures the key features of angina:
a disorder of the breast marked with strong and peculiar symptoms, considerable for the kind of danger belonging to it, and not extremely rare, the sense of strangling and anxiety with which it is attended, may make it not improperly be called angina pectoris. Those who are afflicted with it are seized while they are walking (more especially if it be uphill, and soon after eating), with a painful and most disagreeable sensation in the breast, which seems as if it
would extinguish life, if it were to increase or to continue; but the moment they stand still, all this uneasiness vanishes.
would extinguish life, if it were to increase or to continue; but the moment they stand still, all this uneasiness vanishes.
As observed by William Heberden, angina is “not extremely rare”: it affects approximately 3.1 million men and 3.3 million women in the United States (overall, 3.8% of the population) and there are an additional 400,000 new cases each year (1). The prevalence of angina rises markedly with age (21.1% in men, 13.7% in women 65 to 69 and 27.3% and 24.7%, respectively, for men and women 80 to 84 years of age) (2). Thus, angina is highly prevalent especially in the elderly, has a major impact on lifestyle and quality of life, and imposes a major financial burden on the individual and a huge socioeconomic impact on the community.
Pathophysiology of Angina
Cardiac ischemic pain is transmitted via sensory afferents located in the coronary vessels and myocardium (3). These afferents are sensitive to both stretch and by local expression of specific chemical stimuli (4). Maseri and colleagues (3) categorized cardiac ischemic pain into three components: (a) a diffuse visceral component, (b) a better defined somatic component conforming to a distribution by dermatomes, and (c) an interpretive component modulated by psychological factors. Pain-producing stimuli traveling through afferent nerve endings converge with others from the same dermatome on the same dorsal horn spinal neurons. Cardiac afferents distributed from the first to the fourth thoracic spinal neurons interact with other afferents and descending signals from supraspinal sources, then ascend to the thalamus and from thence to the cortex, where the decoding is processed by a complex collage of physical, emotional, and other factors.
The symptomatic discomfort that represents angina pectoris usually reflects underlying coronary atherosclerosis sufficient to reduce maximal blood flow during exercise (5) (Table 17.1). Whereas fixed segmental coronary stenosis (e.g., >50% diameter stenosis) may prevent sufficient myocardial blood flow to meet the increased oxygen requirements imposed by physical exercise; changes in vascular tone also modulate the threshold for ischemia. Failure of flow mediated dilation is due to deficient endothelial-dependent relaxation (6,7,8) and is associated with the diffuse nature of coronary artery disease, even in the absence of stenotic atheromatous lesions. Thus, angina may result from exercise-induced coronary vasoconstriction of noncritically narrowed coronary arteries and imbalance between oxygen supply and demand.
TABLE 17.1 Causes of Anginal Chest Pain | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mental or physical stress can precipitate angina pectoris and ischemia; mental stress is mediated by sympathetic activation, with a commensurate increase in myocardial oxygen requirements resulting from tachycardia, hypertension, and increased contractility (9). This exerts a double jeopardy on the ischemic myocardium by also reducing regional coronary flow (10). Failure of endothelial-dependent epicardial coronary vasodilatation is evident during mental stress in patients with stable angina (10), and, vasoconstriction of coronary resistance vessels may be present. Several neurohumoral factors contribute including serotonin, neuropeptide Y, norepinephrine, angiotensin II, thromboxane A2, endothelin, and arginine vasopressin (11,12). As many as one of five stable angina patients have features of recent injury and/or repair in their culprit coronary lesions. Aggregation and activation of platelets can contribute to the alterations in vascular tone (13).
Stable Angina: A Symptom Complex
Most patients with stable angina describe retrosternal chest discomfort or distress, rather than “pain.” Anginal discomfort is sometimes characterized as heaviness, burning, tightness, or a choking sensation. It is commonly felt in the center of the chest, characteristically gestured with a clenched-fist or the flat of the hand across the sternum (14). In some patients, it is exclusively located outside the chest, in the arms, shoulders, back, jaw, or epigastrium. Patterns of anginal radiation are associated with severe ischemia and can spread from the chest to the neck, shoulders, arms (usually left), and jaw. Anginal equivalents characterized by dyspnea, profound fatigue, weakness, or syncope may occur in the absence of any discomfort. Ischemic symptoms during stable angina are usually of brief duration, persisting for 3 to 5 minutes, and are typically relieved by rest, dissipation of emotional distress, or the administration of nitroglycerin. Typically, the symptoms are produced by vigorous physical activity or emotional distress, and the threshold at which they occur may be lowered by exposure to cold weather, by smoking a cigarette, or after ingestion of a meal. Some patients experience warm-up angina (possibly a form of ischemic preconditioning) such that they experience angina much more readily on initiating exercise, than after the episode of angina. After pausing for the initial episode of angina to dissipate, they are able to continue for a sustained time at the same or even an accelerated pace. Warm-up angina is evident in approximately 20% of patients; hence, the second exertional effort is predictably better than the first if there is separation of at least
2 to 5 minutes between them (15). However, if the second effort is initiated later than 30 to 60 minutes after the first, the improvement disappears. Marber and colleagues (15), along with others (16,17,18), have suggested a pathophysiologic link triggered by favorable adaptive myocardial metabolic changes that result in less of a decline in high-energy phosphate and less lactate production despite recurrent ischemia. Experimental insights into the phenomenon of ischemic preconditioning have demonstrated changes in mitochondrial KATP channel function, leading to reduced requirement for oxygenated substrate and attendant reduction in myocardial oxygen consumption (18,19).
2 to 5 minutes between them (15). However, if the second effort is initiated later than 30 to 60 minutes after the first, the improvement disappears. Marber and colleagues (15), along with others (16,17,18), have suggested a pathophysiologic link triggered by favorable adaptive myocardial metabolic changes that result in less of a decline in high-energy phosphate and less lactate production despite recurrent ischemia. Experimental insights into the phenomenon of ischemic preconditioning have demonstrated changes in mitochondrial KATP channel function, leading to reduced requirement for oxygenated substrate and attendant reduction in myocardial oxygen consumption (18,19).
Angina is traditionally categorized into four grades based on the Canadian Cardiovascular Society grading scale (20). In class I, patients experience angina only with strenuous or protracted physical activity; those in class II experience only slight limitation with vigorous physical activity such as walking up a hill briskly. Patients in class III have marked limitation, with symptoms during the activities of everyday living, and those in class IV have the inability to perform the activities of daily living because of symptoms as well as angina that may occur at rest. This classification does not address changes in the pattern or frequency of angina (including the development of unstable angina) or take into account the warm-up effect or the self-imposed alteration in activities of daily living that may subtly modify symptomatic status (21).
Silent Ischemia
The chronologic sequence of events during ischemia begins with diminished myocardial perfusion and is followed by diminished diastolic and systolic left ventricular function, abnormal myocardial lactate metabolism, electrocardiographic (ECG) changes, and then finally symptoms of angina pectoris (22,23). Most ischemic episodes in patients with stable angina (>75%) are clinically silent, and despite symptomatic control of angina, a substantial proportion (40% of patients with stable angina) continue to demonstrate ischemia on ambulatory monitoring (22).
Impairment of contractile function may persist for an extended period of time (60–120 minutes after exercise-induced angina) despite abrupt normalization of hemodynamic and ECG parameters. Ambrosio and colleagues (24) demonstrated the delay in return of contractile performance despite normal perfusion after the development and relief of exertional angina (myocardial stunning), in the context of severely obstructive coronary artery disease.
Syndrome X
Cardiac syndrome X represents a heterogeneous group of disorders best characterized by a reduced capacity of the coronary circulation to augment flow in the face of an increase in oxygen demand. Abnormalities of coronary vasomotor tone and angina, or angina-like chest pain may occur in the presence of angiographically normal coronary arteries (25). In addition, about 25% of patients with stable angina have coronary lesions on angiography that do not alter exercise-induced coronary flow (26,27). Evidence for myocardial ischemia in such patients has been demonstrated by reversible perfusion defects with thallium scintigraphy and transient impairment of global and regional left ventricular function by radionuclide ventriculography (28). Survival in patients with syndrome X is not significantly impaired in comparison with age- and gender-matched controls (29).
Prinzmetal’s Variant Angina
Focal coronary spasm has been demonstrated as a mechanism for variant angina based on the association of transient ST elevation concurrent with symptoms and localized myocardial perfusion and functional abnormalities (30,31). This uncommon but well-recognized syndrome (30) is evident in up to 2% of patients presenting with chest pain undergoing invasive study. It is usually associated with underlying fixed coronary obstruction, but a substantial cohort may have angiographically normal coronary arteries or minimally evident disease (32).
Demographics and Outcome of Patients with Angina
Evaluation of a general outpatient population of 5,125 patients with stable angina enrolled by 1,266 primary care physicians in the United States demonstrates approximately equal gender distribution (mean age of women of 71 years and that of men of 67 years) (33). In the Coronary Artery Surgery Study, 62% of women with definite angina had coronary disease compared with a much higher proportion (89%) of men (34). Most patients had more than one cardiovascular-related illness, usually systemic hypertension, hypercholesterolemia, prior infarction, heart failure, or diabetes. The majority perceived their health to be either poor or fair and had experienced at least two episodes of angina per week, and although more than 90% had angina with activity, nearly half also experienced angina at rest, highlighting the commonality of mixed angina. Recently, the 5-year outcome and risk characteristics of a trial population of patients with chronic ischemic heart disease was defined (35). The rate of death, nonfatal myocardial infarction (MI), or stroke varied almost 10-fold according to baseline risk characteristics (from 1% to 9%) (Table 17.2). Thus, estimating baseline risk is critically important in weighing up the balance between risk and benefit of therapeutic interventions.
New-onset angina, defined as occurring within 2 to 3 months of presentation, is associated with at least a doubling of the risk of nonfatal MI within the first year after onset (36). This accentuated risk over patients with chronic coronary disease appears in spite of a lesser extent of triple-vessel disease and a greater frequency of single-vessel disease than in patients with chronic stable angina (37).
African Americans, especially those born in the southern United States, have an excess of cardiovascular mortality compared with American whites (38). Less aggressive use of diagnostic procedures has been recorded in African Americans (39,40). Asian Indians living outside of India have an excess risk of MI (range, 2.5 to 5.0) and mortality for coronary artery disease (range, 1.5 to 3.0) compared with indigenous populations (41). Their disease is characterized by premature onset and a severe and diffuse nature such that it is less amenable to coronary artery bypass grafting (CABG) and more likely to lead to permanent disability. Factors promoting this more malignant course include increased triglycerides and lipoprotein(a) levels, low high-density lipoprotein (HDL) cholesterol levels, insulin resistance, and more prevalent diabetes occurring earlier in life (42). A consistent inverse relationship exists between indicators of socioeconomic status and coronary artery disease (43). Although socioeconomic status is strongly and inversely linked to conventional risk factors such as cigarette smoking, hypertension, cholesterol, and obesity, it is likely to be an independent risk factor for cardiovascular disease.
TABLE 17.2 Risk Factors for Adverse Prognosis in Patients with Chronic Coronary Artery Disease (38) | |
|---|---|
|
Principles of Management
The clinical assessment of patients with angina pectoris should involve a systematic review of cardiac and extracardiac factors that might contribute to the genesis of symptoms. Hence, cardiovascular factors such as hypertension, left ventricular hypertrophy, aortic and other valvular disease, and arteritis must be considered. Important contributory systemic illnesses such as anemia, thyrotoxicosis, renal disease, chronic volume overload, and high-output states need identification. Homocysteinuria (estimated prevalence of 1% to 2% of the population in a heterozygous state) has been found to be associated with symptomatic coronary artery disease (43). In the U.S. Physician Health Study, the adjusted relative risk for disease in the highest 5% versus the lowest 90% of homocysteine levels was 3.4 (95% confidence interval, 1.3–8.8%, P = .01) compared with matched-paired controls (44). However, correction of elevated homocysteine with folic acid does not appear to improve outcome (NORVIT, ESC 2005 Hotline Presentation) (44a).
Identification is required of cardiac and systemic factors that support the clinical suspicion of coronary disease (lipid profiles; hypertension with signs of target organ damage; microalbuminuria; concomitant vascular disease in extracranial neck vessels, abdominal aorta, or peripheral arteries). Left ventricular dysfunction and elevated left ventricular filling pressure may, uncommonly, be accompanied by a presystolic fourth heart sound, cardiomegaly, mitral regurgitation, or paradoxical splitting of the second sound. However, most commonly findings on physical examination of a patient with chronic stable angina are normal unless signs of the contributory illnesses or cardiac complications are present.
Diagnostic Tests
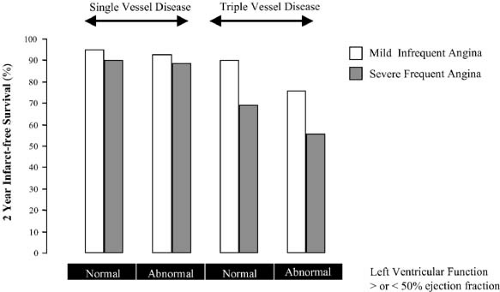
The ECG at rest is commonly normal in patients with angina pectoris, but it can demonstrate evidence of prior infarction or with persisting ST-segment elevation, aneurysm, T-wave change, intraventricular conduction defects, and atrial abnormalities may also be evident. ECG observations captured during an episode of angina permit evaluation of the location and extent of ECG changes. When the total amount of ST-segment change is extensive (>12 mm), there is a high positive predictive accuracy for the detection of three-vessel or left main coronary disease (45). Frequency of angina, especially with dysfunction, predicts three-vessel coronary disease (Fig. 17.1).
The graded exercise stress test forms the cornerstone of diagnostic testing in patients with known or suspected stable angina pectoris. It should be performed in all such patients before undertaking more detailed or invasive procedures. At least four
important potential objectives may be achieved by conducting this test:
important potential objectives may be achieved by conducting this test:
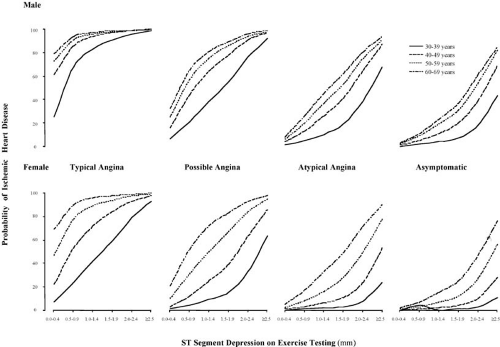
Correlation of patient symptoms with the presence of ischemia. When angina occurs with ischemic ECG changes, the prediction of coronary disease is more reliable (46) but the predictive accuracy is lower in women (Fig. 17.2).
Definition of risk of future events. Those patients who are unable to exercise for more than 6 minutes on the Bruce protocol or demonstrate significant ischemia within this temporal window are at increased risk and merit further investigation (47). A summary of high-risk variables associated with unfavorable prognosis during exercise testing is shown in Table 17.3. A recent addition to this profile is the observation that a delay in the decrease in heart rate during the first minute after a graded exercise test was strongly predictive of mortality at 6 years (19% versus 5%; relative risk of 4.0).
To assess the level of activity and heart rate–blood pressure product at which ischemia develops.
To evaluate the efficacy of pharmacologic and/or revascularization therapy by assessing subjective and objective manifestations of ischemia.
Deriving an index or score from information relating to the duration of exercise, the extent of ST-segment deviation, and the reproduction of limiting or nonlimiting angina can provide a more precise estimation of prognosis.
In various circumstances, more specialized noninvasive assessment is warranted. When an adequate exercise test
is coupled with either myocardial nuclear imaging or two-dimensional echocardiography, it is a highly effective and diagnostically accurate test (Table 17.4). Both exercise and the infusion of dobutamine induce ischemia through an increase in myocardial oxygen consumption mediated by augmented heart rate, systolic blood pressure, and contractility. However, exercise results in a 50% greater increment in heart rate systolic blood pressure product versus dobutamine in direct comparisons, probably accounting for its greater sensitivity in detecting coronary artery disease in some series (48).
is coupled with either myocardial nuclear imaging or two-dimensional echocardiography, it is a highly effective and diagnostically accurate test (Table 17.4). Both exercise and the infusion of dobutamine induce ischemia through an increase in myocardial oxygen consumption mediated by augmented heart rate, systolic blood pressure, and contractility. However, exercise results in a 50% greater increment in heart rate systolic blood pressure product versus dobutamine in direct comparisons, probably accounting for its greater sensitivity in detecting coronary artery disease in some series (48).
TABLE 17.3 Features Associated with a Poor Prognosis and Indicative of Severe Disease | |
|---|---|
|
TABLE 17.4 Relative Advantages and Disadvantages of Three Main Noninvasive Stress Testing Methods | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Given that a high proportion of patients with stable angina have prior infarction and/or wall motion abnormalities at rest detected by two-dimensional echocardiography or resting perfusion defects, the question of whether such wall motion abnormalities possess residual myocardial perfusion and viability becomes highly relevant in therapeutic planning. Dobutamine enhancement of left ventricular dysfunction or inotropic stimulation evident after a ventricular premature beat is predictive of improvement in function with revascularization of the affected segment (49). Delayed imaging, 18 to 24 hours after thallium exercise scintigraphy, reveals that 50% or more of segments with apparently irreversible thallium defects on delayed imaging (at 3–4 hours) demonstrate isotope redistribution thought to be indicative of severe ischemia and predictive of favorable response to revascularization (50). Thallium reinjection at rest, 3 to 4 hours after stress imaging, has been demonstrated to provide similar information to delayed imaging in a more time-efficient and practical fashion (51). The use of thallium in this fashion to detect myocardial viability has been validated by concomitant metabolic imaging using positron emission tomography with oxygen-15–labeled water and exogenous glucose use with fluorine-18–labeled fluorodeoxyglucose (51).
By contrast, dipyridamole- and adenosine-induced hyperemia results in maldistribution of coronary flow by maximally dilating normal vascular segments, whereas those with fixed coronary stenosis already have near-maximal dilatation, resulting in image defects. Adenosine’s rapid onset of action and extremely short half-life, as opposed to that of dipyramidole, circumvents the need for theophylline reversal of troublesome side effects such as bronchial constriction (52). Two-dimensional echocardiography performed before and immediately after exercise testing and continuously before, during, and after dobutamine infusion is used to detect new or worsening preexisting wall motion abnormalities (53,54). These findings show excellent concordance with the territory of the affected coronary vessel (48); they demonstrate prognostic value in patients with known or suspected coronary artery disease (55,56,57).
Perfusion imaging with thallium-201– or technetium-99m–labeled radiopharmaceuticals (either sestamibi [MIBI] or tetrofosmin) provides visualization of myocardial blood flow. Injected immediately before cessation of exercise or pharmacologic stress, these agents can delineate relative differences in myocardial blood flow conforming to areas of myocardial ischemia. With thallium-201, a late image associated with redistribution of blood flow 3 to 4 hours after exercise can be obtained to assess reversibility of defects observed during exercise (58). By contrast, with the technetium-99m–labeled MIBI, myocardial distribution is relatively fixed without significant redistribution; hence, imaging can be conducted for a few hours after the time of injection during pharmacologic or exercise stress (59). A second injection is necessary to characterize blood flow at rest. Perfusion is also useful in assessing ischemia in specific vascular segments when coronary disease has been established (28). Substantial data are available to support the role of myocardial perfusion imaging in more precisely defining prognosis; in this regard, a high-risk scan may be especially useful, and the characteristics of this finding are depicted in Table 17.3 (60,61,62,63,64).
Although coronary calcification has long been recognized as a marker of atherosclerosis, its clinical utility has been limited. The availability of electron beam (ultrafast) tomography has dramatically increased the sensitivity of coronary calcium detection (65). However, there may be marked intrapatient variability on repeat testing and its role remains controversial (66). An American College of Cardiology/American Heart Association consensus document concluded that “the test has proven to have a predictive accuracy approximately equivalent to alternative methods for diagnosing coronary artery disease but has not been found to be superior to alternative non-invasive tests” (67).
Intravascular Imaging
Fiber-Optic Imaging (Angioscopy)
Visualization of the physical appearance of atheromatous lesions, and in particular the detection of unsuspected plaque
rupture events, has provided new information on the diffuse nature of coronary arterial disease. In particular, the detection of multiple “vulnerable” plaques in segments of coronary artery without detectable abnormality on angiography has led to key insights into the mechanisms of progression of acute coronary arterial disease (68). In support, Buffon and colleagues measured myeloperoxidase proximal and distal to suspected unstable plaques (69). These findings suggest multiple sites of plaque disruption with associated platelet aggregation and thrombosis. However, angioscopy remains a research tool and its focus is mainly in the field of acute coronary artery disease rather than stable ischemic syndromes.
rupture events, has provided new information on the diffuse nature of coronary arterial disease. In particular, the detection of multiple “vulnerable” plaques in segments of coronary artery without detectable abnormality on angiography has led to key insights into the mechanisms of progression of acute coronary arterial disease (68). In support, Buffon and colleagues measured myeloperoxidase proximal and distal to suspected unstable plaques (69). These findings suggest multiple sites of plaque disruption with associated platelet aggregation and thrombosis. However, angioscopy remains a research tool and its focus is mainly in the field of acute coronary artery disease rather than stable ischemic syndromes.
Intravascular Ultrasound
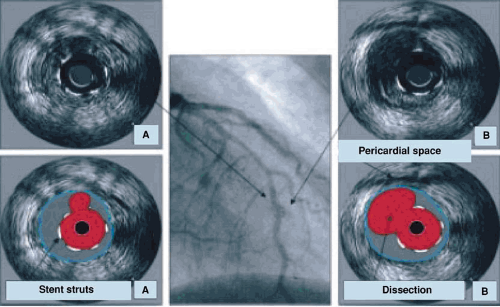
High-resolution intravascular ultrasound (IVUS) has provided critically important observations that demonstrate the extent and distribution coronary artery disease and reveal the severity and eccentric nature of plaque lesions. These may be underestimated on angiography (Fig. 17.3). Vascular remodeling (as originally described by Glagov, 73) results in extensive atheroma but relative preservation of the coronary arterial lumen until late in the disease process.
IVUS has also provided insights into plaque composition, including the distribution and extent of lipid-rich plaques, extent of calcification, and thickness of the fibrous cap (70). All of these features provide evidence of susceptibility of atheromatous plaques to rupture, but validated outcome studies are required to demonstrate that interventions on such nonstenotic lesions alter clinical outcome.
Optical Coherence Tomography
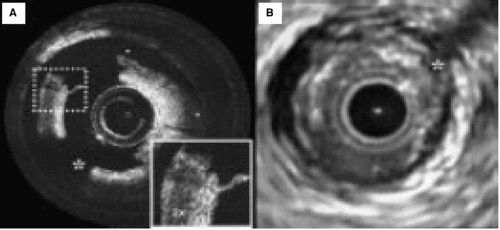
Using light from an intravascular device, and having cleared red cells from the field of view, it is possible to obtain exquisite resolution of structures in the vascular wall (71) (Fig. 17.4). This technique has great potential as an experimental tool, but it may have limited clinical application on account of the need for a blood-free field and the fact that only limited segments of the coronary arterial tree are currently accessible with this device.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is emerging as the reference standard for clinical assessment of ischemia and for evaluation of myocardial viability. The exquisite structural information provided by either MRI (72) (Fig. 17.5A) or multislice CT (73,74). In addition to such structural information, MRI in conjunction with administration of a contrast agent (e.g., gadolinium chelate) can be used to detect perfusion abnormalities and viability (75) (Fig. 17.5B). Perfusion measurements were first investigated based on tissue water content detected by MRI and evaluation of T1 and T2 signals. However, such assessment is not sufficiently reliable without the administration of MRI contrast material (75,76,77,78,79). First-pass imaging in the presence of a contrast agent allows the detection of impaired perfusion and can provide assessment of the physiologic significance of stenoses in different vascular beds.
An alternative approach involves the detection of reversible left ventricular contractile dysfunction using multiphasic MRI
(75). Such regional dysfunction is associated with reversible ischemia (e.g., with dipyridamole stress). Sensitivity, specificity, and accuracy of 91%, 80%, and 90%, respectively, have been reported, and dobutamine stress MRI avoids the problems of poor acoustic windows in patients assessed with dobutamine stress echo.
(75). Such regional dysfunction is associated with reversible ischemia (e.g., with dipyridamole stress). Sensitivity, specificity, and accuracy of 91%, 80%, and 90%, respectively, have been reported, and dobutamine stress MRI avoids the problems of poor acoustic windows in patients assessed with dobutamine stress echo.
In addition to assessing the volume of myocardial flow affected by decreased perfusion, late enhancement of gadolinium chelate allows the detection of reperfused but viable myocardium and the potential for distinguishing such zones from myocardium with impaired perfusion but without the potential for function recovery (79). Thus, MRI imaging with the aid of MRI contrast agents can detect abnormalities in structure and function of the ventricle, estimate the volume of myocardium with reduced perfusion, and demonstrate potentially viable segments. For these reasons, MRI is emerging as the reference standard for the assessment of the impact of functional stenoses in coronary arteries.
Coronary Angiography
Selective coronary angiography is the most widely used diagnostic investigation to define the extent and severity of intrinsic coronary narrowing. In general, it should be performed in patients (a) when the diagnosis of coronary disease is important to establish yet remains in doubt after noninvasive assessment, (b) when high-risk coronary disease is suspected based on the results of clinical and noninvasive evaluation, and (c) in non–high-risk patients when significant symptoms persist despite adequate medical therapy or medical therapy is poorly tolerated.
Coronary angiography is widely applied, but significant limitations need to be considered:
Angiographically apparently “normal” vessels may have extensive nonstenotic coronary disease, as demonstrated histopathologically or with high-resolution IVUS.
The physiologic importance of moderate and apparently similar stenoses may differ markedly (as demonstrated by measurements of coronary flow reserve) (80,81).
Standard coronary angiography does not assess the impact of changes in vascular tone.
As a result of collateral development, angiographic appearances may underestimate or overestimate the impact of stenosis on myocardial perfusion.
The angiogram does not provide insights into the histologic structure of stenotic lesions or the likelihood of plaque rupture.
Disrupted endothelium or minor plaque fissures may precipitate signs and symptoms of non–ST-elevation acute coronary syndrome and yet may be difficult to detect on coronary angiography.
Despite these limitations, coronary angiography provides key information on the extent and distribution of coronary stenoses and long-term survival (Fig. 17.6). It may be combined with provocative testing with ergonovine maleate in patients with known or suspected Prinzmetal variant angina and may identify the location and extent of provokable coronary spasm (82). In patients without major anatomic narrowing and in the absence of Prinzmetal variant angina, vasoreactivity of lesions may be provoked by intravenous ergonovine in almost 20% of patients. Combined with IVUS to provide precise definition of not only the extent of coronary narrowing and the thickness and extent of atheroma in the coronary arterial wall (83,84).
Therapeutic Interventions
In chronic stable coronary disease, the goals of treatment are to relieve symptoms and reduce the risk of future cardiac events (including acute coronary syndrome, heart failure, and death) (Fig. 17.7). Simultaneous rather than alternative approaches are required: these include lifestyle modification, management
of risk factors, pharmacologic therapy, and coronary revascularization. All forms of intervention present some hazard and hence they should be instituted when the perceived benefits, in terms of improved symptoms and prognosis, outweigh the associated risks. The clinician, and the informed patient, need to institute an overall treatment strategy to minimize the impact of the disease throughout the remainder of the patient’s life. Accurate assessment of prognostic risk is critically important in this regard.
of risk factors, pharmacologic therapy, and coronary revascularization. All forms of intervention present some hazard and hence they should be instituted when the perceived benefits, in terms of improved symptoms and prognosis, outweigh the associated risks. The clinician, and the informed patient, need to institute an overall treatment strategy to minimize the impact of the disease throughout the remainder of the patient’s life. Accurate assessment of prognostic risk is critically important in this regard.
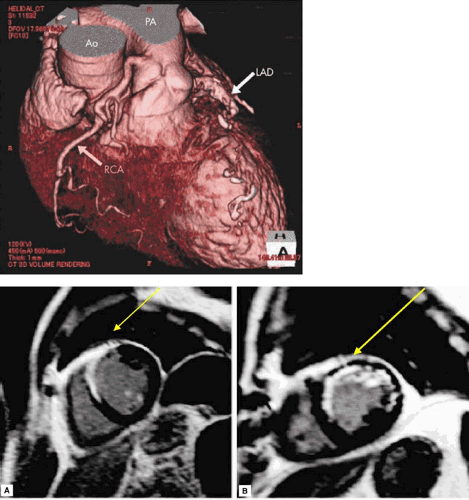 FIGURE 17.5. (upper panel) Imaging to define anatomy: contrast-enhanced multidetector computed tomography. (Source: Kobayashi K, Tokunaga T, Isobe M. A case of anomalous of the right coronary artery from the pulmonary artery complicated by acute myocardial infarction. Heart 2005;91:1130 ). (lower panels A, B) Imaging to detect MI. Contrast enhanced magnetic resonance imaging. (A) Transmural anteroseptal infarction with hyperenhancement shown in white. (B) Nontransmural infarction extending from septum to lateral wall (arrows) (79). |
Lifestyle and Risk Factor Modification
Lifestyle and risk factor modifications are integral to treatment of patients with chronic stable coronary disease. They may provide both symptomatic and prognostic benefits and their impact may enhance mechanical or pharmacologic treatment
and their lack of impact may substantially diminish potential benefits.
and their lack of impact may substantially diminish potential benefits.
 Get Clinical Tree app for offline access 
|