Coronary Angiography
Deepak L. Bhatt
Frederick A. Heupler Jr.
Overview
Coronary angiography remains the clinical gold standard for the diagnosis of coronary artery disease. Approximately 2 million procedures are performed annually in the United States. More than 60% of cardiologists in the United States perform coronary angiography as part of their practice (1). Because it is an invasive procedure with potentially serious risks, coronary angiography should be performed only by well-trained individuals when an appropriate clinical indication exists. Physicians who perform coronary angiography must clearly understand its limitations and possess a firm grasp of the fundamental technical aspects of catheterization (2).
Glossary
Caudal projection
Radiographic view with the image intensifier pointing to the inferior surface of the heart.
Conus artery
A branch that variably arises either from the right coronary artery or in close proximity to it and perfuses the right ventricular outflow tract.
Cranial projection
Radiographic view with the image intensifier pointing to the superior surface of the heart.
Crux
The intersection of the atrioventricular groove and the interatrial and interventricular septa on the inferior aspect of the heart.
Damping
Blunting of a recording of the arterial pressure waveform.
Diagonal artery
A branch of the left anterior descending artery that perfuses the left ventricular free wall.
Dominance
Reference to the vessel, either the right coronary artery or the left circumflex artery, that supplies the posterior part of the heart; in codominance, both arteries provide circulation to the posterior heart.
Image intensifier
The portion of the catheterization laboratory imaging equipment that increases the brightness of images produced by x-rays. Image intensifier units are being replaced in many catheterization laboratories with a newer technology, flat detector units.
Internal mammary artery (IMA)
An artery that runs from the subclavian artery down the chest wall that can be harvested for use as a surgical conduit for bypass grafting; also called the internal thoracic artery.
Left anterior descending (LAD) artery
The artery that supplies the anterior surface of the heart.
Left anterior oblique (LAO) projection
Radiographic view with the image intensifier on the left side of the patient.
Left circumflex artery (LCx)
The artery that supplies blood to the lateral aspect of the heart.
Left main (LM) coronary artery
The short artery that divides into the left anterior descending artery and the left circumflex artery (and sometimes the ramus intermedius) and supplies most of the left side of the heart.
Marginal artery
A branch (acute marginal) of the right coronary artery that supplies the right ventricle; also, a branch (obtuse marginal) of the left circumflex, sometimes referred to as a lateral branch.
Posterior descending artery (PDA)
The branch of the right coronary artery, or occasionally the left circumflex artery, that provides blood flow to the posterior aspect of the interventricular septum.
Ramus intermedius (RI)
A branch of the left main artery that supplies the lateral wall of the left ventricle.
Right anterior oblique (RAO) projection
Radiographic view with the image intensifier on the right of the patient.
Right coronary artery (RCA)
The artery that supplies blood to the right side of the heart and usually to the posterior aspect of the left ventricle.
Saphenous vein graft
A surgical conduit harvested from the legs that is used to reroute blood from the aorta to the coronary arteries.
Scatter
Dispersion of radiation as it passes through the body; it is the source of most of the radiation exposure to the operator.
Ventricularization
Distortion of the recorded arterial pressure waveform such that the diastolic component is blunted, simulating a left ventricular pressure tracing.
General Principles
Historical Perspective
The first selective coronary angiogram was performed in 1958 by Dr. F. Mason Sones, Jr., a cardiologist at the Cleveland Clinic Foundation in Ohio (3). When Dr. Sones and Dr. Earl K. Shirey published their results of more than 1,000 procedures in 1962, interest in coronary angiography surged. Coronary angiography as a diagnostic tool blossomed with Dr. René Favaloro’s description in 1968 of his pioneering work in performing saphenous vein coronary artery bypass grafting at the Cleveland Clinic.
Radiologists played an important role in the development of catheterization techniques in the early 1960s. New preformed catheter designs, such as those by Dr. Melvin Judkins and Dr. Kurt Amplatz, enabled selective angiography to be performed with greater ease than was previously possible with the Sones catheters. Additionally, percutaneous approaches were also now possible, and arterial cutdowns were no longer required. Improvements in radiographic imaging concomitantly led to better image quality. After Dr. Andreas Grüntzig introduced percutaneous coronary angioplasty in 1977, cardiologists were able to make the transition from being diagnosticians to becoming endovascular surgeons.
Coronary Artery Anatomy
Normal Anatomy
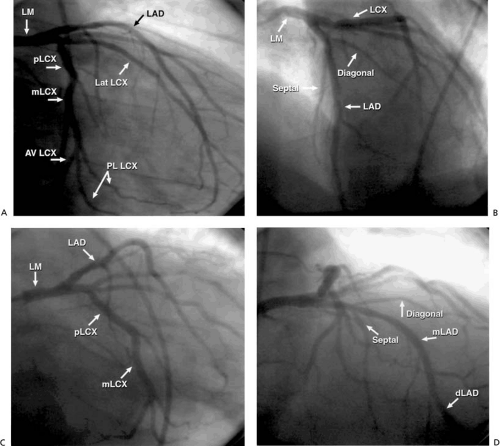
The left main (LM) coronary artery originates from the left sinus of Valsalva and usually bifurcates into the left anterior descending artery (LAD), which supplies the left ventricle’s anterior surface, and the left circumflex artery (LCx), which supplies the lateral aspect of the left ventricle (Fig. 76.1). The LAD traverses the anterior interventricular groove, giving rise to septal perforating branches to supply the interventricular septum and to diagonal branches that supply the anterolateral wall. It then bifurcates distally and tapers out as a “whale’s tail” at the cardiac apex, although sometimes it wraps around the apex to supply part of the inferior wall. The LCx courses along the left atrioventricular groove and provides atrial branches to the left atrium and marginal branches that supply the lateral wall of the left ventricle. The marginal branches are sometimes referred to as lateral branches, with the first marginal branch called the high lateral and subsequent lateral branches referred to as lateral or posterolateral branches. Occasionally, the LM coronary artery trifurcates to give rise also to a ramus intermedius branch that supplies the high lateral wall of the left ventricle.
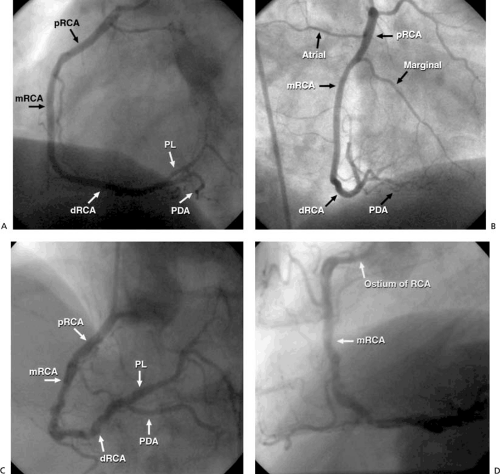
The right coronary artery (RCA) originates from the right sinus at a slightly more caudal level than the LM coronary artery and supplies the right ventricle and the inferior aspect of the left ventricle (Fig. 76.2). It traverses the right atrioventricular groove and provides small branches to the right atrium and marginal branches to the right ventricle. Approximately 60% of the time, the RCA atrial branches supply the sinus node; otherwise, the LCx atrial branches serve this function. Approximately 50% of the time, the first branch of the RCA is a conus branch, supplying the right ventricular outflow tract; the remainder of the time, the conus branch originates separately from an ostium near the RCA ostium. The dominant artery is generally defined as the one that provides the posterior descending artery (PDA) to supply the posterior wall. In approximately 85% of patients, the RCA is dominant, with the remainder of patients having either a dominant LCx or a codominant RCA and LCx. The PDA of the RCA (or the LCx) courses along the inferior interventricular groove, providing septal perforators, a feature that may aid in its identification and differentiation from the posterolateral segment of the RCA (or LCx), which gives off branches to the posterior ventricle. More than 90% of the time, the artery to the atrioventricular node originates from the atrioventricular branch of the RCA as it passes through the crux.
Coronary Anomalies
It is important for the angiographer to be alert for possible variations in normal coronary anatomy. In a series of 126,595 patients undergoing coronary angiography at the Cleveland Clinic Foundation, Yamanaka and Hobbs (4) found coronary artery anomalies in 1.3% of patients. Of the anomalies identified, 87% involved anomalies of origin or distribution, 13% involved the presence of coronary artery fistulae, and 81% were considered benign. (Table 76.1). Some anomalies may be potentially harmful, such as a coronary artery that originates from the pulmonary artery or the opposite aortic sinus. For example, most patients with an anomalous origin of the left coronary artery (LCA) from the pulmonary artery (Bland-White-Garland syndrome) die as infants unless surgical repair is undertaken.
Origin of the LCA from the right sinus may also lead to sudden death, depending in large part on the traversal of the anomalous artery. If the anomalous vessel travels intramyocardially through the septum (the most common variant of this anomaly), or anteriorly or posteriorly, symptoms of ischemia are very unlikely. If the anomalous artery courses between the pulmonary artery and aorta, however, exercise may precipitate angina or sudden death. In this latter variant, the LM coronary artery projects on end as a “dot” on the anterior surface of the aorta during a ventriculogram or aortogram in an right anterior oblique (RAO) projection. Likewise, a single coronary artery or large coronary artery fistulae may be problematic. Patients with large coronary artery fistulae may present with heart failure or ischemia, and these lesions may also cause endocarditis; therefore, endocarditis prophylaxis is recommended (5). Small coronary artery fistulae, however, are rarely problematic. These fistulae typically originate from the LAD and drain into the pulmonary artery two thirds of the time, with drainage into a cardiac chamber in the remainder of instances.
Approximately 10% of patients with coronary artery anomalies have some form of congenital heart abnormality, such as a bicuspid aortic valve (6). In tetralogy of Fallot, for example, the LAD sometimes originates from the right sinus, crossing the right ventricular outflow tract. Communication between cardiologist and cardiac surgeon is important to convey the presence of any coronary anomalies and thus prevent any accidental transection or ligation of anomalous coronary arteries (7).
Myocardial Bridging
Systolic compression of a coronary artery is referred to as bridging. Approximately 12% of normal angiograms demonstrate
some degree of myocardial bridging, although only 1.7% show systolic narrowing of more than 50% (8). Bridging occurs in the mid-LAD when it courses through the septum. If this is the case, it is important to note this for the surgeon, because it makes grafting more technically challenging, especially from a minimally invasive approach. Myocardial bridging of septal perforating branches, also called septal squeeze, is commonly found in hypertrophic cardiomyopathy and aortic stenosis and occasionally with severe proximal LAD stenosis, but rarely in normal hearts (9). Other more unusual locations for bridging include the PDA. When a circumflex marginal branch is intramyocardial, ventricular systole usually produces little or no compression. The intramyocardial portion appears unusually straight rather than serpentine. Nitrate administration tends to make bridging more apparent angiographically. Because the majority of coronary flow occurs in diastole, the systolic phenomenon of bridging rarely leads to ischemia (8). In the few instances in which it does lead to objective signs of ischemia, stenting has been performed successfully (10,11,12,13).
some degree of myocardial bridging, although only 1.7% show systolic narrowing of more than 50% (8). Bridging occurs in the mid-LAD when it courses through the septum. If this is the case, it is important to note this for the surgeon, because it makes grafting more technically challenging, especially from a minimally invasive approach. Myocardial bridging of septal perforating branches, also called septal squeeze, is commonly found in hypertrophic cardiomyopathy and aortic stenosis and occasionally with severe proximal LAD stenosis, but rarely in normal hearts (9). Other more unusual locations for bridging include the PDA. When a circumflex marginal branch is intramyocardial, ventricular systole usually produces little or no compression. The intramyocardial portion appears unusually straight rather than serpentine. Nitrate administration tends to make bridging more apparent angiographically. Because the majority of coronary flow occurs in diastole, the systolic phenomenon of bridging rarely leads to ischemia (8). In the few instances in which it does lead to objective signs of ischemia, stenting has been performed successfully (10,11,12,13).
Collateral Circulation
When an occlusion or severe narrowing of a coronary artery develops, collateral channels may form from a nearby artery to supply blood to the hypoperfused area of myocardium. Collaterals form between arteries that occupy adjacent areas of the heart, and such connections cannot form if a cardiac chamber is superimposed in between. For example, collateral development via septal perforators is common between the LAD and PDA when one of these vessels is occluded. Occasionally, a separate conus branch arising near the RCA supplies collaterals to the LAD; identification is important if consideration is being given
to bypass surgery targets. Kugel artery is a small vessel that arises from the proximal RCA or LCx, or both, and traverses the interatrial septum; it commonly terminates near the crux, where it may act as a source of collaterals to the distal RCA or LCx via the atrioventricular nodal artery. Intracoronary collaterals, so-called bridging collaterals, may form from the same coronary artery across an area of total vessel occlusion. Identification of such bridging collaterals and recognition of an underlying chronic total occlusion are important. Although rare, pericardial or bronchial arteries may provide coronary collaterals (14). When collaterals are present from more than one source, competitive flow from both sources may occur, leading to inadequate opacification of the target vessel; this may produce the false appearance of a significant luminal obstruction at the point where the two collateral flows meet.
to bypass surgery targets. Kugel artery is a small vessel that arises from the proximal RCA or LCx, or both, and traverses the interatrial septum; it commonly terminates near the crux, where it may act as a source of collaterals to the distal RCA or LCx via the atrioventricular nodal artery. Intracoronary collaterals, so-called bridging collaterals, may form from the same coronary artery across an area of total vessel occlusion. Identification of such bridging collaterals and recognition of an underlying chronic total occlusion are important. Although rare, pericardial or bronchial arteries may provide coronary collaterals (14). When collaterals are present from more than one source, competitive flow from both sources may occur, leading to inadequate opacification of the target vessel; this may produce the false appearance of a significant luminal obstruction at the point where the two collateral flows meet.
Coronary Artery Spasm
Prinzmetal angina or variant angina refers to the syndrome of coronary artery spasm that causes transient ST-segment elevation during angina (15,16). This may result in anginal chest pain at rest, often in the early morning, as well as syncope from heart block or ventricular arrhythmias. The coronary arteries of these patients are predisposed to spasm, often despite the absence of angiographically severe atherosclerosis. Prinzmetal angina is rare, and it is recognized clinically even less often than in the past owing to the advent of calcium channel blockers and long-acting nitrates (17). In patients with no significant atherosclerosis but a suspicion of spasm, Holter monitoring may reveal ST-segment shifts that verify the diagnosis. If angina occurs concurrent with ST-segment elevation in a
patient with a “normal” catheterization, the diagnosis is made, with no need for confirmatory provocative testing.
patient with a “normal” catheterization, the diagnosis is made, with no need for confirmatory provocative testing.
TABLE 76.1 Relative Frequency of Coronary Artery Anomalies | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Provocative testing for coronary artery spasm is most likely to be clinically useful in the patient with no severe coronary artery obstructions, in whom it has been impossible to document the diagnosis. It is not likely to be useful in patients with fixed severe coronary obstructions, and the risk of testing is increased in these individuals. The major risk of provocative testing is unrelenting spasm, with subsequent myocardial infarction. Therefore, when this test is performed, intraarterial nitroglycerin and calcium channel blockers should be immediately available. The method to perform provocative testing involves administration of 0.05 mg intravenous methylergonovine while monitoring a complete electrocardiogram every minute (18,19). If typical chest pain or electrocardiographic changes develop, the coronary arteries should be reimaged immediately. Five minutes after the methylergonovine has been administered, if there has been no chest pain or electrocardiographic changes, the coronary arteries should be reimaged. If there is no evidence of spasm, 0.2 mg methylergonovine should be administered and the foregoing procedure repeated. Some operators prefer to omit the 0.05-mg dose to save time. If spasm does occur, 100 to 200 μg nitroglycerin should be administered intraarterially to relieve the spasm. Intracoronary acetylcholine is another provocative agent for coronary spasm, but it probably lacks the specificity of methylergonovine.
Diffuse spasm that occurs without angina or ST-segment changes may be physiologic and is not diagnostic of Prinzmetal angina. Methylergonovine-induced severe coronary spasm may recur after immediate treatment because of the longer half-life of methylergonovine compared with nitroglycerin; therefore, the patient should be observed and treated with systemic long-acting nitrates and calcium channel blockers (20). Provocative testing should be avoided in patients with severe systemic or pulmonary hypertension, as well as in patients who may be pregnant. Spasm may also be a cause of chest pain or myocardial infarction after acute alcohol ingestion or cocaine use, in which case the clinical history is useful in making the diagnosis (21). Nonischemic, “catheter-induced” coronary spasm, especially ostial spasm, may be triggered by the catheter tip.
Coronary Artery Dissection
Rarely, spontaneous coronary artery dissection produces an ischemic syndrome or sudden death (22). Predisposing factors include pregnancy, a burst of intense physical exertion, and cocaine use (23,24). Pregnant or postpartum women are the demographic group in which spontaneous coronary artery dissection is most commonly seen, and the LAD is the vessel most frequently involved (25). Severe hypertension may also predispose to spontaneous dissection (26). Although not exactly spontaneous, blunt trauma, such as from a motor vehicle accident, may also lead to dissection (27). Aortic dissection may also extend into the coronary arteries, particularly the RCA. Marfan syndrome may be complicated by coronary dissection, as may connective tissue disorders such as Ehlers-Danlos syndrome. Arteritis, such as that seen in polyarteritis nodosa, may lead to dissection (28). Additionally, dissection may occur as a result of catheter trauma. It is important to recognize this entity, which may appear as a radiolucent flap in the opacified artery or as a linear persistent stain in the arterial wall after the contrast material has cleared from the true lumen.
Regardless of the cause, dissection can often be treated percutaneously with stent deployment (29,30). Although healing of dissection with conservative treatment has been reported, this approach is less common in the interventional era (31). In some instances of postpartum dissection, immunosuppressive therapy has been used with success (32). Eosinophils appear to be present in the dissection plane in cases of peripartum dissection, similar to peripartum cardiomyopathy, a finding suggesting an inflammatory origin in at least some instances (33). The plane of dissection is often between the media and adventitia of the vessel, although in some cases, an intimal tear is noted at autopsy. Some cases of spontaneous dissection, especially in older individuals at risk for atherosclerosis, are probably the result of plaque rupture and intimal dissection (34).
Coronary Artery Ectasia and Aneurysm
Coronary artery ectasia refers to an abnormal enlargement of the coronary arteries. Arteries that are ectatic appear to be prone to thrombus formation, dissection, and spasm (35). Additionally, prior percutaneous intervention occasionally leads to development of segments of ectasia. When the area of dilatation is more than 1.5 times the diameter of the normal portion of the artery, the enlarged portion is referred to as an aneurysm (36). The RCA is the vessel most frequently involved. Atherosclerosis is the most common cause of coronary ectasia and aneurysm (37). Kawasaki disease leads to formation of giant coronary artery aneurysms in approximately 20% of untreated patients (38,39,40,41). Mycotic aneurysms of the coronary arteries are rare (42). Historically, large coronary artery aneurysms have been treated conservatively with antiplatelet or anticoagulant therapy, or both, but, more recently, surgery and intracoronary stent graft deployment have been used (43,44). When there is associated stenosis, invasive treatment may be indicated.
Indications
Coronary angiography is indicated when the coronary artery anatomy needs to be delineated. The American College of Cardiology (ACC) has published guidelines to assist the clinician
in appropriate use of this invasive procedure (45) (Table 76.2). Indications are listed as class I when there is general consensus that angiography is indicated, class II when opinions diverge, and class III when the consensus is that angiography should not be performed. In a patient with anginal chest discomfort and evidence of ischemia on a functional study, coronary angiography may reveal a stenosis that is amenable to angioplasty or coronary artery bypass surgery (class I). In a patient with an acute coronary syndrome, angiography is given a class II indication. However, emerging evidence supports an invasive approach in the initial evaluation of patients with acute coronary syndromes (46,47). The development of pharmacologic adjuncts, such as the glycoprotein IIb/IIIa inhibitors, has enhanced the safety of percutaneous intervention and has broadened the indications for cardiac catheterization. Thus, the ACC guidelines provide a framework for the practice of evidence-based medicine but do not substitute for clinical judgment, and they do not necessarily incorporate the most current research.
in appropriate use of this invasive procedure (45) (Table 76.2). Indications are listed as class I when there is general consensus that angiography is indicated, class II when opinions diverge, and class III when the consensus is that angiography should not be performed. In a patient with anginal chest discomfort and evidence of ischemia on a functional study, coronary angiography may reveal a stenosis that is amenable to angioplasty or coronary artery bypass surgery (class I). In a patient with an acute coronary syndrome, angiography is given a class II indication. However, emerging evidence supports an invasive approach in the initial evaluation of patients with acute coronary syndromes (46,47). The development of pharmacologic adjuncts, such as the glycoprotein IIb/IIIa inhibitors, has enhanced the safety of percutaneous intervention and has broadened the indications for cardiac catheterization. Thus, the ACC guidelines provide a framework for the practice of evidence-based medicine but do not substitute for clinical judgment, and they do not necessarily incorporate the most current research.
TABLE 76.2 Indications for Coronary Angiography | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
TABLE 76.3 Relative Contraindications to Coronary Angiography | |
|---|---|
|
Contraindications
The only absolute contraindication to coronary angiography is patient refusal. According to the ACC classification, indications that are labeled as class III are actually situations in which coronary angiography is contraindicated (Table 76.2). Additional relative contraindications are shown in Table 76.3. Relative contraindications imply that in certain urgent clinical circumstances, proceeding with angiography may be appropriate, although caution must be exercised.
Operator Proficiency
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree