CHAPTER 70 Type A Aortic Dissection
HISTORICAL NOTE
The observations by Morgagni in 1761 were followed by multiple early anatomic and postmortem reports describing aortic dissection, including the famous autopsy report on King George II of England in 1776.1 In 1802, Maunoir, describing this disease, used the term dissection.2 Almost 20 years later, René Laennec coined the term aneurysme dissequant, or dissecting aneurysm, believing that this entity represented the early stage of a saccular aneurysm.3 Later, in 1863, Peacock published a comprehensive review of 80 cases of aortic dissection.4 Until the last half of the 20th century, the diagnosis of aortic dissection was almost exclusively an autopsy finding. Antemortem diagnosis was made in only 6 of the 300 cases reviewed by Shennan in 1934.5 The use of contrast angiography for the diagnosis of aortic dissection was reported by Paullin and James.6 The first attempt to treat this condition was described in 1935 by Gurin and colleagues,7 who used surgical iliac artery fenestration to relieve lower extremity ischemia. Although quickly abandoned, cellophane wrapping of the dissected aorta was also attempted to prevent rupture.8 In 1955, DeBakey and associates9 launched the modern era of surgical management with graft replacement of the dissected aorta. Subsequently, the same group introduced the use of cardiopulmonary bypass during clamping of the descending thoracic aorta.10 The first large clinical series of aortic dissection was published in 1958 by Hirst and colleagues11; analysis of the findings in 505 cases allowed these authors to emphasize the high mortality rate and the glaring rarity of antemortem diagnosis at that time. The modern medical approach to aortic dissection with use of pharmacologic agents to diminish aortic dP/dt (anti-impulse therapy) was introduced by Wheat and Palmer and associates in 1965.12 The venerable DeBakey classification of aortic dissection was described the same year; the simplified Stanford classification system (type A or type B) based on pathophysiologic characteristics was proposed in 1970 by Daily, Shumway, and colleagues.13,14 These developments were followed by other important advances, such as less invasive and more accurate diagnostic modalities, improved anesthetic methods, safer extracorporeal perfusion techniques, profound hypothermic circulatory arrest for thoracic aortic arch surgery introduced by Griepp and coworkers15 from Stanford in 1975, improved and safer prosthetic vascular grafts, and refinement of cardiovascular surgical techniques.
CLASSIFICATION
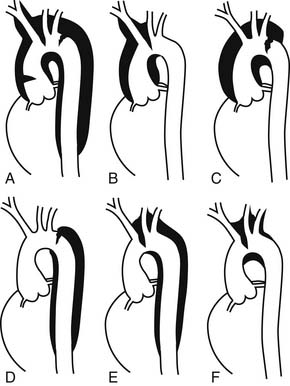
It is important to understand and to apply accurately the classification of aortic dissection to treat patients most appropriately and also to compare rigorously the results of various medical and surgical therapeutic interventions reported from different institutions. Considerable confusion has arisen in the past in classifying aortic dissections. Numerous systems have been proposed, beginning in 1955 with the nine categories initially suggested by DeBakey and associates.9 The more widely used DeBakey type I, type II, and type III three-category classification scheme was introduced in 196514; importantly, DeBakey modified this scheme in 198216 to comply with the Stanford A/B functional criteria based on whether the ascending aorta is involved regardless of the location of the tear. Despite the use of different labels, a consensus has emerged concerning the essential elements of a common functional classification system of aortic dissection. The key point of all classification systems used today is the presence or absence of involvement of the ascending aorta, regardless of the location of the primary intimal tear and irrespective of the antegrade extension of the dissection process.17 The simplified Stanford classification approach as proposed by Daily and associates13 in 1970 has gained broad acceptance during the past 30 years. If the dissection involves the ascending aorta, it is a Stanford type A, which corresponds to a DeBakey type I,16 University of Alabama “ascending,”18 Massachusetts General Hospital “proximal,”19 and Najafi “anterior” dissection.20 Both DeBakey type I and type II dissections involve the ascending aorta; type I extends beyond the innominate artery, whereas type II is confined just to the ascending aorta. If the ascending aorta proximal to the innominate artery is not involved in the process, the dissection is called Stanford type B, DeBakey type III, descending, distal, or posterior (Fig. 70-1), even though many of these patients have some limited extent of retrograde dissection involvement in the arch. This point is commonly not appreciated. A subtype of dissection in which the primary intimal tear is in the descending aorta or even farther distal, yet the dissection process propagates in a retrograde fashion to involve the arch and ascending aorta, was originally termed DeBakey type III-D by Reul and colleagues21 in 1975 but now is simply termed a retro-A dissection. Retro-A dissections constitute about 6% of all type A dissections.22
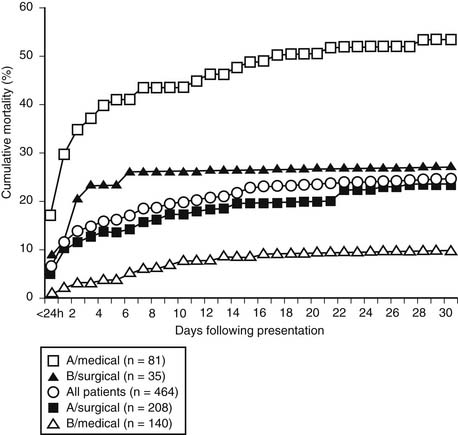
Aortic dissections diagnosed within 14 days of the onset of presenting symptoms are defined as acute; those diagnosed more than 14 days after onset are classified as chronic dissections.23–25 According to the International Registry of Acute Aortic Dissection (IRAD) investigators,24 the cumulative mortality after acute type A and type B dissection treated medically reached a plateau after the 14th day following presentation, demonstrating the prognostic importance of this venerable—but arbitrary—14-day time distinction (Fig. 70-2). DeBakey and colleagues16 introduced the term subacute to describe dissections between 2 weeks and 2 months old, but this distinction is rarely used today.
During the past 2 decades, advances in vascular imaging technology have led to increasing recognition of intramural hematoma (IMH) and penetrating aortic ulcers as distinct pathologic variants of classic aortic dissection.26,27 Both are characterized by the absence of the classic intimal flap dividing the aorta into true and false channels. IMH can be precipitated by an atherosclerotic ulcer penetrating into the internal elastic lamina or can occur spontaneously without any intimal disruption. IMH can involve the ascending aorta (type A IMH) as well as the descending aorta (type B IMH). On occasion, IMH can evolve suddenly into a classic aortic dissection with blood flow in both lumens,28 and they may be different phases of a dynamic pathologic process. Penetrating atherosclerotic ulcers occur most commonly in the descending thoracic aorta. Distinguishing IMH (with or without a penetrating aortic ulcer) or penetrating aortic ulcers from classic aortic dissection is critical because the pathophysiologic process, clinical behavior, prognosis, and management of these lesions can differ,29–31 depending on which segment of the aorta is involved and the patient’s symptoms.
EPIDEMIOLOGY
Aortic dissection is seen in all age groups, although the majority of the cases occur between the ages of 50 and 69 years. In a series of 464 patients reviewed in the IRAD, mean age was 63 years.24 Two thirds of aortic dissections involve the ascending aorta (Stanford type A) and a third involve the descending aorta (Stanford type B).24 Typically, patients with type B dissection are older than those with type A dissection.10,24 Dissection in patients younger than 40 years typically is a type A dissection. According to the IRAD investigators, young patients with acute aortic dissections are less likely to be hypertensive and are more likely to have Marfan syndrome, bicuspid aortic valve, and prior aortic surgery.32 In all studies, there is a clear male predominance with an estimated male-to-female ratio ranging from 2:1 to 3:1. Although less frequently affected by acute aortic syndromes, women are older at the time of diagnosis with a mean age of 67 years.24 Hirst and colleagues11 found a higher incidence of aortic dissection in African Americans, which might be related more to hypertension than to any intrinsic racial pathologic weakness of the aorta that increases the probability of aortic dissection. A high prevalence of hypertension may also explain why the incidence of aortic dissection is higher in Japan.
The exact incidence of aortic dissection has been difficult to determine because many patients die without the correct diagnosis being made antemortem.33 It is not widely appreciated that acute aortic dissection is the most common clinical catastrophe involving the aorta. Moreover, its incidence has been increasing in the industrialized world, in part because of the increasing life expectancy and longer exposure to elevated blood pressure. In a 1964 Danish study of 6480 autopsies covering 90% of a regional population, the incidence of acute aortic dissection was 5.2 per million population per year, higher than the incidence of ruptured abdominal aortic aneurysm (3.6 per million population per year) and more than four times the prevalence of ruptured thoracic aortic aneurysm (1.2 per million population per year).34 In the seminal 1958 pathologic series by Hirst and associates,11 acute aortic dissection was found in 1% to 2% of autopsies. In the 1970s, it was estimated that the incidence of acute aortic dissection in an urban population in the southeastern United States might be as high as 10 to 20 cases per million population per year,35 or approximately 2000 to 4500 new cases each year.36 According to a review of all thoracic aortic diseases among the residents of Olmsted County, Minnesota, between 1980 and 1994, the annual incidence of acute aortic dissection was estimated at 3.5 per 100,000 persons.33 More recently, in a comprehensive review of the entire Swedish national health-care registry during a 15-year period, the incidence of thoracic aortic aneurysm and dissection had increased substantially between 1987 and 2002; more specifically, the incidence of thoracic aortic disease rose by 52% in men and by 28% in women during this period to reach 16.3 cases per 100,000 per year and 9.1 cases per 100,000 per year, respectively.37 Prevalence figures undoubtedly underestimate the real incidence because they do not consider patients who die suddenly of a complication of aortic dissection and who are presumed to have succumbed to coronary disease or an arrhythmic event in the absence of a postmortem examination; importantly, this was not the case in the report from Sweden, where many dissections were identified only at the time of forensic autopsy, which is still performed almost universally in that country. Most physicians tend to think that ruptured abdominal aortic aneurysms are more common; this misconception comes from the fact that ruptured abdominal aortic aneurysms are diagnosed correctly more often than are acute aortic dissections.
NATURAL HISTORY
According to the historical autopsy analyses, untreated acute aortic dissection is a highly lethal event. In the study by Shennan published in 1934,5 40% of patients with dissection involving the ascending aorta died immediately, 70% within the first 24 hours, 94% within the first week, and 100% within 5 weeks. In 1967, Lindsay and Hurst38 reported that one third of patients sustaining an acute aortic dissection died within 24 hours, 50% within 48 hours, 80% within 7 days, and 95% within the first month. In patients presenting with chronic dissection, only 15% were still alive after 5 years. In patients with dissection involving the descending thoracic aorta (Stanford type B), 25% had died 1 month after onset. Later, Anagnostopoulos and coworkers,39 in a large collected series of 963 cases of untreated patients with aortic dissection of all types, reported a cumulative mortality of 70% at 1 week and 90% at 3 months.
Most patients with untreated acute type A dissection die of intrapericardial rupture culminating in cardiac tamponade; other causes of death include acute aortic valvular regurgitation resulting in left ventricular failure, coronary ostial compromise causing acute myocardial ischemia, occlusion of aortic branches supplying the cerebral or visceral circulation, and free aortic rupture. Patients with untreated acute type B dissection usually die of aortic rupture or of distal end-organ malperfusion (occlusion of major aortic branches resulting in ischemic injury to vital abdominal organs), or thoracoabdominal malperfusion.23 In the Stanford experience, lower extremity ischemia at presentation did not significantly increase surgical mortality risk, whereas occlusion of major abdominal tributaries resulting in renal or splanchnic ischemia was associated with very high mortality rates.40,41
Only 10% of acute dissections are estimated to “heal” spontaneously, eventually becoming chronic dissections; in nearly all cases, distal reentry sites are found, allowing decompression of the false lumen.42,43 After acute aortic dissection, the false lumen usually remains patent but very rarely may thrombose, depending mainly on the presence, size, and site of distal false lumen reentry sites. When the false lumen remains patent, it will eventually be prone to progressive expansion over time, resulting in the formation of a false aneurysm. As a general rule of thumb, a large fraction of serious or fatal dissection complications are caused by a non-reentering false lumen, which compromises distal blood flow by extrinsically narrowing or occluding the true lumen.
PREDISPOSING FACTORS
Arterial Hypertension
In patients with aortic dissection, the prevalence of arterial hypertension varies between 45% and 80%,10,23,24,43,44 being highest in patients with acute type B dissection. Untreated arterial hypertension promotes medial smooth muscle cell degeneration and other changes in the aortic wall, which may increase the susceptibility for aortic dissection.45 Although there is no evidence to suggest that hypertension initiates the actual process, it is a major risk factor.
Connective Tissue Disorders
Heritable connective tissue disorders such as Marfan, Ehlers-Danlos, and Loeys-Dietz syndromes are associated with an increased risk of aortic dissection. The Marfan syndrome, described initially by Antoine Bernard Marfan in 1896, is inherited as an autosomal dominant trait and is characterized by mutations of the FBN1 gene, situated on the long arm of chromosome 15 and encoding the glycoprotein fibrillin 1, which is a major component of elastic fibers of the extracellular matrix in various organs.46,47 In addition to cardiovascular manifestations, including mitral valve prolapse, progressive aortic dilation, aortic valve regurgitation, and aortic dissection, these patients can have several other ocular and musculoskeletal abnormalities, integument and pulmonary disorders, and dural ectasia. Because the genetic testing available currently is still unreliable and very complex, the diagnosis of Marfan syndrome today is made on clinical grounds according to the revised Ghent criteria (major and minor), which characterize the involvement of different organ systems.48 When a patient presents with the classic Marfan syndrome phenotype, the diagnosis is rarely in doubt; however, phenotypic expression of Marfan syndrome can be extremely variable, and many patients have only some of the characteristic features, including aortic root dilation or anuloaortic ectasia with or without aortic valve regurgitation, which has been called the forme fruste of Marfan syndrome.46 Aorta-related complications, including acute dissection and rupture, are the leading causes of death in patients with Marfan syndrome.49 If a patient with Marfan syndrome has a family history of aortic dissection or other aortic catastrophe at an early age, the risk of dissection or rupture is considerably higher.47,50 The prevalence of Marfan syndrome based on large series of aortic dissection ranges between 5% and 12%.10,24,44,51
Patients with Ehlers-Danlos syndrome, particularly those with type IV Ehlers-Danlos syndrome, which is transmitted in most cases as an autosomal dominant trait, have arterial weakness of all large and muscular arteries; type IV Ehlers-Danlos syndrome is characterized by a procollagen type III abnormality and an increased risk of aortic dissection or spontaneous rupture of peripheral arteries or a hollow abdominal viscus.52 Aortic rupture has also been reported in patients with Ehlers-Danlos syndrome type I and type VI. Extremely fragile arteries are found in patients with Ehlers-Danlos syndrome, and vascular procedures, including simple arterial puncture and suture repair, can be fraught with devastating complications.53 Familial clusters of aortic dissection linked to an autosomal dominant trait have also been reported and could also be related to a mutation in the gene coding for type III procollagen.54,55
Loeys-Dietz syndrome is a recently recognized autosomal dominant connective tissue disorder characterized by premature arterial aneurysms (typically aortic root aneurysm) and aortic dissection along with diffuse peripheral arterial tortuosity. It is caused by heterogeneous mutations in the genes encoding transforming growth factor β receptors 1 and 2.56,57 Phenotypic features of the syndrome include hypertelorism, bifid uvula or cleft palate or both, and generalized arterial hypertortuosity. In these patients, the risk of aortic rupture and aortic dissection exceeds that of most known connective tissue disorders. Also, aortic rupture and dissection can occur at a younger age (even in infants) and at smaller aortic diameters.
Congenital Abnormalities
Congenital heart problems, such as bicuspid aortic valve and coarctation of the aorta, are associated with an increased risk of aortic dissection compared with that in the general population. In an analysis of 186 autopsies of patients who died of type A aortic dissection, it was found that the prevalence of unicuspid or bicuspid aortic valves was 9%.58 The risk of perioperative and late postoperative dissection is also increased in patients with a bicuspid aortic valve after any type of cardiac surgery, especially in patients with dilation of the ascending aorta.59,60 Aortic dissection predominantly involves the ascending aorta in patients with coarctation,61 and the dissection may not propagate beyond the aortic isthmus. Simultaneous management of an acute type A aortic dissection in patients with uncorrected aortic coarctation is challenging and may require modification of the CPB arterial cannulation strategy and occasionally the use of an extra-anatomic thoracic aortic graft to bypass the coarctation.62,63
Iatrogenic Injury
Aortic dissection is a rare complication of cardiac catheterization and other percutaneous diagnostic and therapeutic interventional techniques involving manipulation of catheters inside the thoracic aorta.64 Catheter and guide wire injuries are usually self-limited, localized subintimal dissections that only rarely require surgical intervention. On the other hand, life-threatening iatrogenic dissections can occur during endovascular device deployment (aortic stent graft or transfemoral percutaneous aortic valve replacement) or open surgical procedures; among 7000 cardiac operations, iatrogenic aortic dissection complicated 0.3% of cases.65 These can be related to ascending aortic cannulation, retrograde dissection after femoral artery cannulation, aortic crossclamp or partial occluding clamp injury, and intimal injury at the site of a proximal bypass graft anastomosis.64,66 Surprisingly, the incidence of iatrogenic perioperative type A aortic dissection has been noted to be increasing in patients undergoing off-pump coronary artery bypass, which was attributed to multiple ascending aortic manipulations for proximal anastomoses67; various proximal vein graft mechanical connectors have also been associated with dissection.
Pregnancy
In women younger than 40 years, approximately 50% of dissections occur during the third trimester or during labor and delivery; a substantial proportion of these patients have an identifiable heritable connective tissue disorder, such as Marfan syndrome, or a bicuspid aortic valve associated with a dilated ascending aorta.68,69 The hemodynamic and hormonal alterations of pregnancy, culminating in the third trimester, are thought to be the causes of dissection in susceptible individuals.
Illicit Drug Related
One of the cardiovascular complications of cocaine use, particularly crack cocaine inhalation, is acute aortic dissection.70,71 Aortic dissection in this setting occurs presumably as a consequence of abrupt, severe hypertension and catecholamine release, and this diagnosis should be considered in cocaine or methamphetamine abusers presenting with chest pain.
Associations
Aortic dissections also occur more frequently in patients with Turner’s syndrome and Noonan’s syndrome.72 Infrequent associations include giant cell aortitis, systemic lupus erythematosus, Cushing syndrome, pheochromocytoma, polycystic kidney disease, familial hypercholesterolemia, and relapsing polychondritis.42,43
PATHOPHYSIOLOGY AND PATHOLOGIC FINDINGS
Medial Degeneration
Erdheim73 in 1929 was the first to describe what he called cystic medial necrosis, a nonspecific pathologic process involving medial smooth muscle cell loss, elastic lamellar disruption, and acid mucopolysaccharide accumulation within the aortic media. This abnormal architecture is believed to lead to changes in the distribution of both circumferential wall stress and shear stress in the aortic media, potentially leading to an intimal tear.74 It is now known that the word cystic is a misnomer because these medial lesions do not form true cysts (they are not lined by epithelial cells). The term necrosis is also misleading. In young patients (particularly those with a heritable connective tissue disorder), the elastic elements of the aortic media are disrupted and disorganized; in older individuals, it is the smooth muscle elements of the aortic media that are abnormal because of aging and hypertension75,76 and probably represent changes associated with repeated aortic wall injury and repair. Thus, “cystic medial necrosis” should be replaced by more specific terms relating to alterations of the elastic fibers (“elastic type”) and smooth muscle cells (“smooth muscle type”) in the media in younger and older patients, respectively. In patients with inherited connective tissue disorders, such as Marfan syndrome, pathologic examination of the aortic wall frequently revealed pronounced medial degeneration, with severe loss of elastic lamellae and accumulation of mucoid substance within the media. These young patients typically present with an acute type A dissection. On the other hand, in older individuals, type B dissections are more common and are associated with medial degeneration characterized by loss of smooth muscle cells. Recently, it was proposed that the coexistence of activated T lymphocytes and macrophages as well as markers of apoptotic vascular cell death in the aortic media of patients with aortic dissection may contribute to the two pathways leading to medial degeneration, namely, elimination of vascular smooth muscle cells and degradation of the extracellular matrix.77 Increased contents of collagen types I and III as well as increased activity of connective tissue growth factor have been observed in the media and adventitia of patients with aortic dissection78; these phenomena are likely to be responsible for the reduced aortic distensibility and compliance. Another contributing factor to progressive degradation of the extracellular matrix is excessive activation of matrix metalloproteinases, particularly matrix metalloproteinase 9, which are zinc-dependent proteolytic enzymes that can disrupt the balance composition of vascular smooth muscle cells and extracellular matrix proteins.79 Specific nucleotide polymorphism of the gene encoding matrix metalloproteinase 9, more specifically, the presence of the −8202A/G allele, seems to be associated with an increased risk of aortic dissection.80
Primary Intimal Tear
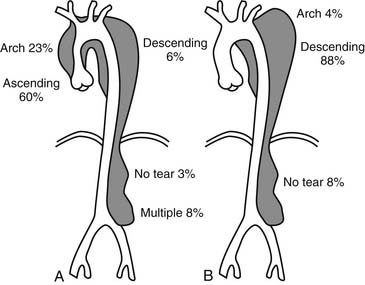
Most authors believe that the initiating event in aortic dissection is a tear in the intima allowing blood to enter the aortic wall and culminating in progressive separation of the medial layers of the aorta and propagation of the dissecting hematoma. The primary intimal tear allows communication between the true aortic lumen and the false lumen. Only 2% to 4% of aortic dissections do not have an identifiable primary intimal tear and are usually confined to the descending thoracic aorta.11 Rarely, an intimal disruption can be present without extensive undermining of the intima and without any false lumen,81 or what we colloquially call intimal stretch marks; if they are associated with localized hematoma within the wall, they have a localized “mushroom cap” appearance. Whether an intimal tear is the precipitating event in all aortic dissections is still debated.74 IMH due to rupture of the vasa vasorum is another potential but infrequent initiating event leading to frank dissection. Rupture of the intima usually happens at points of maximal wall stress along the thoracic aorta. Intimal tears are usually transverse in orientation and typically involve one half to two thirds of the aortic circumference. In rare circumstances, total disruption of intimal continuity with a complete circumferential tear may lead to intimo-intimal intussusception and mechanical obstruction of blood flow82 due to gross prolapse of the circumferential intima. In type A dissections, the majority of intimal tears (60% to 70%) are located in the ascending aorta, usually just distal to the sinotubular junction (Fig. 70-3).11,83,84 The second most common site is the proximal third of the descending aorta, near the aortic isthmus; in 10% to 20%, the intimal tear is located in the aortic arch, usually on the lesser curvature.85,86 In less than 5% of cases, the intimal tear can be located in the abdominal aorta, and the dissection either will be confined to the abdominal aorta or will propagate in a retrograde fashion to involve the thoracic aorta.87,88
Propagation and Reentry
Within the aortic wall, the false lumen is situated between the inner two thirds and the outer one third of the aortic media. On pathoanatomic examination, the dissected aorta is a false aneurysm, because the aortic intima containing the true lumen is not dilated and actually is smaller than normal. Once initiated, aortic dissections usually propagate antegrade or “downstream” but may also extend in a retrograde direction. The dissection often proceeds in a spiral fashion along the aorta. Propagation of the dissection depends on several factors, including rate of increase of aortic systolic pressure or aortic dP/dt, magnitude of aortic diastolic elastic recoil, mean arterial pressure, and aortic wall integrity and strength.42,43,89,90 The mainstay of medical treatment of aortic dissection, as described by Wheat and Palmer in 1965, is directed at reducing aortic dP/dt, or anti-impulse therapy.12 In the ascending aorta, the false lumen usually occupies the right anterior portion; in the arch, the false lumen usually is located along the greater curvature and may extend into the innominate, left carotid, or left subclavian arteries. In the descending and abdominal aorta, the false lumen most often runs along the anterior and lateral aortic walls, frequently incorporating the left renal artery.11 Distal progression of the dissection may be limited by extensive atherosclerosis or anatomic constraints such as aortic coarctation, infrarenal abdominal aortic aneurysm, or abdominal aortic graft anastomosis.61 Otherwise, in young individuals, the dissection almost always involves the entire thoracic and abdominal aorta and extends into the iliac arteries. In patients surviving the acute episode, the false lumen will usually remain patent but rarely may thrombose spontaneously. The presence of distal reentry sites contributes to persistent patency of the false lumen. Partial or complete thrombosis of the false lumen may allow “healing” of the aorta; conversely, if the false lumen reenters distally and stays patent, it is prone to progressive false aneurysmal enlargement. Persistent patency of the distal false lumen is observed in up to 90% of patients after surgical repair of acute type A dissections and may be an adverse prognostic factor associated with a higher incidence of late false aneurysmal degeneration.91,92 Reentry sites are usually multiple and frequently occur at the ostia of sheared off branches, such as the intercostal, visceral, renal, or iliac arteries. Reentry into the true lumen, described by Peacock in 1843 as “an imperfect natural cure of the disease,”1 allows decompression of the false lumen and is the rationale behind surgical and percutaneous flap fenestration techniques.93,94
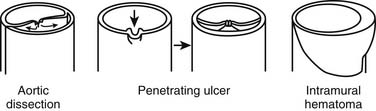
Intramural Hematoma
In 1920, Krukenberg95 first described aortic IMH as a “dissection without intimal tear.” IMH is believed to originate from rupture of vasa vasorum within the outer third of the media, resulting in the circumferential accumulation of blood, as illustrated in Figure 70-4, with no apparent intimal defect visualized on imaging studies.29 IMH may occur spontaneously in predisposed individuals (e.g., elderly and hypertensive patients) or may be a secondary phenomenon after the rupture of an atheromatous plaque through the internal elastic lamina and the formation of a penetrating atherosclerotic ulcer allowing extravasation of blood into the aortic wall, as shown in Figure 70-4.27 The natural history of these lesions was not well characterized until recently. The evolution of IMH may be either benign, with a stable clinical course and eventual healing, or a progressive, often fatal disease, with extension, evolution into classic aortic dissection, aneurysmal degeneration, or aortic rupture.28–3096 In recent years, it was recognized that IMH involving the ascending (type A IMH) or descending (type B IMH) aorta may have a clinical course different from that of classic aortic dissection, especially if it is detected incidentally in asymptomatic patients. Conversely, those presenting with acute severe chest pain are more prone to disease progression and aortic rupture, especially in the presence of an associated deep penetrating ulcer.26,30,96,97 We believe that IMH of the ascending aorta usually has a malignant prognosis comparable with that of acute type A aortic dissection and should be treated accordingly.
Aortic Branch Compromise
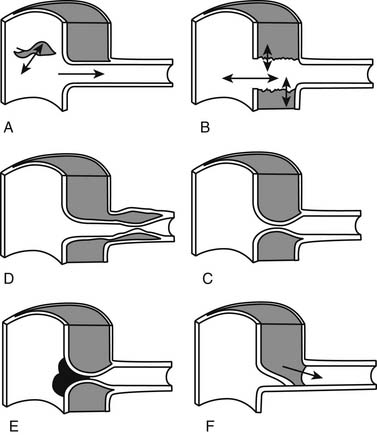
Peripheral branch vessel ischemia or malperfusion arises when the dissection process compromises blood flow to various aortic tributaries. Acute aortic dissection may result in aortic branch compromise through several mechanisms, including extrinsic compression of the true lumen by the pressurized false lumen (particularly a false lumen that does not reenter), an intimal flap compromising the orifice of the branch artery (Fig. 70-5), and occlusion of the tributary if the dissection continues into the branch because of false lumen extrinsic compression of the true lumen distally. A useful pathophysiologic classification of aortic branch compromise was proposed by Williams and associates98: static obstruction occurs when the dissection flap extends into a branch vessel, resulting in mechanical compromise of flow; dynamic obstruction occurs when the dissection flap narrows the aortic true lumen above the branch because of a large false lumen or when the flap prolapses into the vessel origin. As the dissection often progresses in a spiral fashion along the aorta, some aortic branches may be spared and continue to be perfused by the true lumen, whereas other arteries may be perfused exclusively from the false lumen after being sheared off; in this latter scenario, the affected branch subsequently becomes permanently dependent on the false lumen for perfusion after healing of the ostial intimal flap. In some cases, compression by the false lumen may almost eliminate the entire aortic true channel (true lumen collapse or obliteration) with resultant severe distal thoracoabdominal malperfusion.98,99 The pattern of branch artery involvement and the degree of compromise of perfusion determine the clinical presentation, which is enormously variable and often leads to delay before the correct diagnosis is established. In the large autopsy series by Hirst and colleagues,11 the most commonly affected aortic branches were the iliac arteries, followed (in descending order) by the innominate, left common carotid, left subclavian, coronary, renal, superior mesenteric, and celiac axis.
CLINICAL MANIFESTATIONS
Patient Characteristics
Patients with type A dissections are usually younger, including those with Marfan syndrome and other inherited connective tissue disorders or congenital aortic valve disease, whereas type B dissection is usually seen in middle-aged and elderly men. Either type of dissection may occur in women, usually at an older age, but is exceptionally rare in children and teenagers.10,24,100 Older patients usually have coexisting conditions, including hypertension and generalized arteriosclerosis, as well as associated medical comorbidities, such as cerebrovascular, cardiac, pulmonary, and renal disease.
Acute Type A Dissection
Pain
The most common presenting symptom of aortic dissection is severe chest pain usually originating in the anterior chest or in the interscapular region, which correlates with the location of the dissection. The onset of pain is typically abrupt, and the pain is severe at onset, described as sharp or tearing, and is thought to be due to stretching of the aortic adventitial nerve fibers by the dissection. Persistence or migration of the pain suggests continuing expansion within the chest or extension more distally. Differentiation of the chest pain associated with acute aortic dissection from other causes such as acute myocardial ischemia, pulmonary embolism, or pericarditis is critical in the initial evaluation of these patients to allow prompt management. In some patients, the initial pain will disappear either spontaneously or with institution of medical treatment; recurrence of pain thereafter is an ominous sign suggesting impending aortic rupture or continuing downstream extension of the dissection.101 On occasion, acute dissection can be painless. A high clinical index of suspicion is required for aortic dissection as its manifestations can mimic any other acute medical or surgical illness. In a report from IRAD, pain of abrupt onset was the presenting symptom in 85% of patients, and the most common site was the chest. It was located anteriorly in 71% of patients with type A dissection, but 47% and 22%, respectively, of these patients reported back pain and abdominal pain.24
Systemic Manifestations
One third to one half of all patients with acute aortic dissection demonstrate signs and symptoms secondary to cardiac and other organ system involvement.10,24,42,43,102,103 Aortic branch compromise, aortic rupture or leak, and compression of adjacent organs by an expanding false lumen are the most common mechanisms responsible for complications of aortic dissection.
Cardiovascular Manifestations
Patients with acute aortic dissection appear pale and poorly perfused peripherally but generally have elevated blood pressure due to preexistent arterial hypertension, high circulating levels of catecholamines, or renal artery compromise by the dissection. Blood pressure must be recorded in both upper extremities, and a thorough evaluation of peripheral pulses in all four extremities is essential. Hypotension in patients with acute dissection suggests cardiac tamponade104 or impending rupture; shock is usually due to intrapericardial rupture with tamponade, intrathoracic rupture, or acute left ventricular failure secondary to myocardial ischemia or acute, severe aortic valve regurgitation. Aortic rupture is the most frequent cause of death in the acute setting11; the most common site of rupture is near the site of the primary intimal tear.90 Ascending aorta and arch involvement can cause rupture into the pericardial cavity or the mediastinum; however, rupture of a more distal thoracic aortic segment is also possible with exsanguination into the left pleural space or rarely the retroperitoneum. Aortic valve regurgitation is present in 20% to 50% of patients with acute type A dissection.10,23–25,105 Extension of the dissection retrograde into the aortic root with shearing off of one or more aortic valve commissures causes diastolic prolapse of the leaflets. A murmur of aortic regurgitation is heard in 25% to 45% of patients and may be associated with an S3 gallop and pulmonary rales.24,42 Acute, severe aortic regurgitation leading to profound left ventricular failure is the second most common cause of death in patients with type A dissection, after aortic rupture. Less commonly, proximal extension of the dissection into the coronary artery ostia can impair coronary perfusion and cause myocardial ischemia or infarction.106 In this situation, involvement of the right coronary artery is more common than involvement of the left main coronary artery, and the electrocardiographic findings may include changes consistent with acute inferior myocardial infarction. Contained or frank aortic rupture or, more commonly, transudation of fluid from the false lumen through the intact aortic epicardial layer (there is no adventitia because the ascending aorta is an intrapericardial structure) into the pericardial cavity can lead to pericardial effusion and cardiac tamponade associated with jugular venous distention, paradoxical pulse, or pericardial friction rub. Cardiac tamponade is reported in 10% to 20% of patients with acute type A dissection and mandates emergency surgical intervention.104 More rarely, the expanding false lumen may compress surrounding structures, such as the pulmonary artery or the superior vena cava, or rupture into one of the cardiac chambers, resulting in an aortoatrial or aortoventricular fistula.107,108 Heart block can be seen in cases with involvement of the membranous and interatrial septum by a dissection-related hematoma.109
Peripheral Vascular Complications
Systemic arterial manifestations of aortic dissection are the result of the propagation of the dissection process, leading to aortic branch compromise and ischemia or infarction of various end organs. Approximately 30% of patients present initially with symptoms related to acute peripheral arterial compromise or will develop such complications.16,102,103,110,111 Clinical manifestations include coma, stroke, paraplegia, upper or lower extremity ischemia, and anuria or abdominal pain due to renal or mesenteric ischemia.
In a historical review of the Stanford University experience with management of aortic dissection associated with acute peripheral vascular complications by Fann and associates,103 85 (31%) of 272 patients with all types of dissection had one or more peripheral arterial manifestations; 128 had an acute type A dissection, and 40 had an acute type B dissection. Among these 168 patients, 4% presented with acute carotid occlusion and stroke, 5% had acute paraplegia secondary to spinal cord ischemia, 33% sustained loss of one or more peripheral pulses, 11% had impaired renal perfusion demonstrated angiographically, and 6% had compromised visceral perfusion by angiography. The prevalence of these complications according to type of dissection and the operative mortality after definitive intrathoracic surgical management are summarized in Table 70-1. Those with advanced intra-abdominal ischemia or infarction have a dismal prognosis, but simple loss of a peripheral pulse is not a portent of high postoperative mortality or morbidity.
Table 70–1 Peripheral Vascular Complications and Associated Operative Mortality Rates Among 128 Patients with Acute Type A Aortic Dissection
| Peripheral Vascular Complication | Prevalence (n) | Mortality (n) |
|---|---|---|
| Stroke | 6% ± 3% (7) | 14% ± 14% (1) |
| Paraplegia | 6% ± 3% (7) | 43% ± 19% (3) |
| Pulse deficit | 38% ± 5% (48) | 25% ± 6% (12) |
| Renal ischemia | 12% ± 4% (15) | 53% ± 13% (8) |
| Visceral ischemia | 6% ± 3% (8) | 50% ± 18% (4) |
Prevalence and operative mortality ±70% confidence limits; n, number of patients.
Data from Fann JI, Sarris GE, Mitchell RS, et al. Treatment of patients with aortic dissection presenting with peripheral vascular complications.
Acute stroke or transient ischemic attack with hemiplegia is the most common neurologic manifestation. This results from cerebral hypoperfusion secondary to aortic arch involvement or obstruction of the true lumen in a carotid artery. Stroke or transient ischemic attack usually occurs in patients with an acute type A dissection, and the reported incidence ranges from 3% to 7% in large series.16,102,111,112 Beside stroke, altered cerebral perfusion may lead to altered or fluctuating mental status, coma, or syncope in upward of 12% of patients.24 Extensive dissection can compromise perfusion of the spinal cord by shearing off critical intercostal arteries, thereby interrupting flow to the radicularis magna artery and causing paraplegia or paraparesis in 2% to 6% of cases.16,102,103 Symptoms related to involvement of peripheral nerves, such as paresthesia and Horner’s syndrome, are due to peripheral ischemic neuropathy or to direct compression of a nerve by the expanding false lumen. In rare circumstances, stroke, paraplegia, or syncope without chest pain may be the only manifestation in cases of aortic dissection.113
The incidence of renal ischemia due to acute dissection varies from 5% to 25%.43,102,103,111 This wide variability is probably related to methods of detection (ultrasonography, computed tomography, angiography versus autopsy) more than to true population differences. Dissection resulting in renal artery compromise may be asymptomatic and remain undetected unless diagnostic imaging studies are performed but commonly is associated with oliguria or anuria and worsening renal function. Other clinical manifestations of impaired renal perfusion include refractory arterial hypertension, flank pain, and hematuria. In contrast to previous reports observing that the left renal artery is more frequently involved,11 the right renal artery was more often compromised in our experience103; nonetheless, the left renal artery is more frequently fed by the aortic false lumen than by the true lumen.
Aortic dissection involving the visceral arteries leads to mesenteric ischemia or infarction, which is a highly lethal complication. Compromised splanchnic perfusion clinically is relatively uncommon, occurring in less than 5% of cases, although autopsy studies suggest that dissection involvement of the celiac or superior mesenteric arteries is present in more than 10% of patients.11,103 At Stanford, the incidence of angiographically documented visceral ischemia was 6% in patients with acute type A dissection. Visceral ischemia portends a grave prognosis, with mortality rates reported as high as 88%,102,103 but it can have various clinical presentations ranging from asymptomatic angiographic evidence of visceral hypoperfusion to frank bowel infarction. The cardinal association of abdominal pain out of proportion to the findings on physical examination should prompt serious and prompt consideration of mesenteric ischemia.
Peripheral pulse loss occurs in 30% to 50% of patients with acute type A dissection and 25% of all patients irrespective of dissection type.103 The clinical course of patients with peripheral limb ischemia is highly variable and dynamic; therefore, frequent comprehensive pulse examinations are important. In the Massachusetts General Hospital experience, one third of these patients experienced either spontaneous resolution of the pulse deficit or a fluctuating clinical picture.102 This phenomenon is thought to be related to redirection of flow into the true lumen from the false lumen through a spontaneous flap reentry site when the false lumen previously was non-reentering and compromising the true lumen. Alternatively, loss of a peripheral pulse can be asymptomatic, especially in the upper extremities. Rarely, proximal descending thoracic aortic obstruction due to “true lumen collapse” or dynamic “true lumen obliteration” can cause severe ischemia of the entire lower body.99
Chronic Type A Dissection
Patients surviving the initial acute phase of acute dissection, surgically treated or not, will be at risk for development of aortic complications in the chronic phase of the disease. Most patients with chronic type A dissection are asymptomatic until they develop problems related to progressive expansion of the downstream aortic false lumen with aneurysmal degeneration, development of severe aortic valve regurgitation, aneurysmal degeneration of preserved sinuses of Valsalva, or false aneurysms from the previous surgical graft anastomoses. It has been estimated that up to one fourth of patients will develop a downstream aortic false aneurysm and require operation within 10 years after an acute dissection, emphasizing the importance of comprehensive follow-up care and serial imaging studies.16,91,114,115 Some patients who had an asymptomatic acute dissection may present months to years later with a thoracic aortic false aneurysm discovered incidentally on a chest radiograph or a computed tomographic (CT) scan done for an unrelated problem. Progressive expansion of the false lumen may produce compression, obstruction, or erosion into adjacent mediastinal structures. Therefore, symptoms related to aortic aneurysmal enlargement can include chest pain, dyspnea, wheezing or stridor, hoarseness, dysphagia, superior vena cava syndrome, hemoptysis (aortobronchial or aortopulmonary fistula), and hematemesis (aorto-esophageal fistula). Signs and symptoms of heart failure may result if the degree of aortic regurgitation becomes severe. Rarely, late thrombosis of the false lumen may compromise flow in a critical branch perfused solely by the false lumen, resulting in late complications such as paraplegia, lower extremity ischemia, new-onset renal failure, refractory arterial hypertension, or abdominal angina (visceral ischemia). Late aortic rupture can occur into the pericardium, bronchi, esophagus, or pleural cavity, causing tamponade or exsanguination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree