Type A Aortic Dissection after TEVAR
NICOLAS H. POPE and GORAV AILAWADI
Presentation
A 72-year-old male with a history of hypertension presented to the emergency department complaining of persistent chest pain radiating to the back. CT angiography (CTA) demonstrated a chronic type B aortic dissection with aneurysmal dilatation to 5.8 cm without visceral involvement. He underwent endovascular descending aortic repair (EVAR) with an endograft without complication (Fig. 1). He was seen in clinic and found to be doing well without endoleak or further dilation of his aneurysm. He presented to the emergency department 1 year after his procedure complaining of sudden-onset, persistent chest pain that did not worsened activity. He denied shortness of breath or leg swelling. His heart rate was 110 beats per minute with a blood pressure of 140/80 mm Hg. He was afebrile with an oxygen saturation of 98% on room air and a respiratory rate of 18. His physical examination was unremarkable, and he had 2+ peripheral pulses equally in all four extremities.
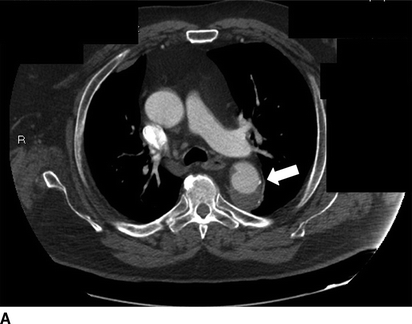
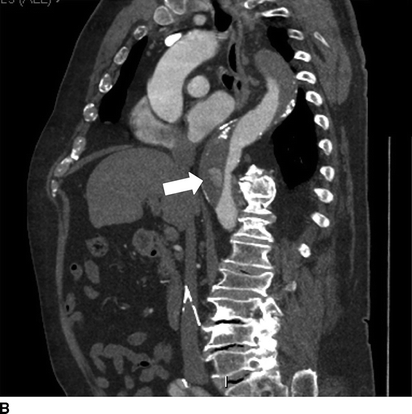
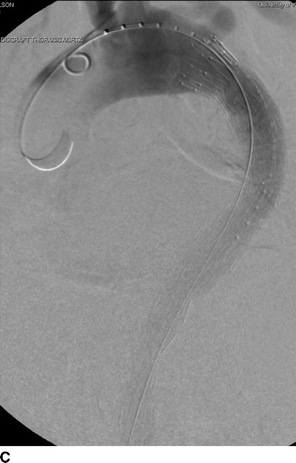
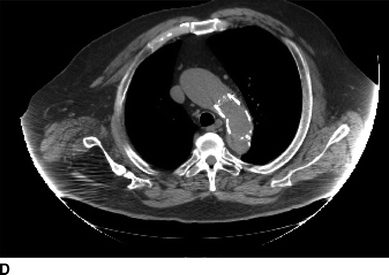
FIGURE 1 Previous type B aortic dissection seen on CTA (arrows) in axial (A) and coronal (B) views. Completion angiography after placement of a Cook Talent TX2 endograft with no evidence of endoleak (C). Noncontrast CT obtained in follow-up demonstrated good position of proximal stent graft without aortic pathology (D).
Differential Diagnosis
The differential diagnosis includes endoleak causing expansion of the existing false aortic lumen with or without contained aortic rupture, myocardial infarction, pulmonary embolism, and type A aortic dissection. Given the patient’s history of type B dissection, further expansion of the false aortic lumen must be high on the differential diagnosis. This may be the result of type I or type II endoleak and may be accompanied by (contained) rupture of the aneurysmal portion of the aorta. A new type A aortic dissection must also be considered. This may be the result of a de novo tear in the ascending aorta or may represent a retrograde dissection related to his previously placed stent graft.
Myocardial infarction (MI) must also be evaluated in this patient. In a patient with a history of aortic dissection, a type A dissection with coronary involvement must also be considered if evidence of myocardial ischemia is present. Pulmonary embolism is unlikely, but should be considered in the differential of acute-onset chest pain.
Workup
History should determine the timing and location of pain and any associated symptoms that may indicate malperfusion. Physical examination should attempt to identify heart murmurs, as new aortic valve insufficiency (AI) as a result of type A dissection or new mitral insufficiency as a result of papillary muscle rupture following MI. Neurologic deficits may indicate arch vessel involvement of a type A dissection.
Workup should include standard laboratory assessment. Cardiac enzymes and an electrocardiogram (EKG) are necessary to rule out myocardial ischemia/infarction. A bedside transthoracic echocardiogram can identify wall motion abnormalities resulting from ischemic areas of myocardium and can often visualize the ascending aorta to evaluate for dissection.
In a hemodynamically stable patient with a thoracic endograft, CTA should be performed early, and is the gold standard to evaluate the position of the graft and look for endoleak and expansion of the false aortic lumen. Chest, abdomen, and pelvis CTA also allows for visualization of the entire aorta and provides important anatomic information to allow for preoperative planning should an aortic repair be required. Ideally, CTA may include the neck to look for extension in the carotid arteries and should be carried as far inferiorly as the femoral vessels to assess their adequacy to support peripheral cannulation (for type A repair) or femoral access for additional stent graft procedures (for endoleak).
Case Continued
Basic laboratory values and cardiac enzymes were normal. EKG showed no ischemic changes, and bedside transthoracic echocardiogram demonstrated no wall motion abnormalities or valvular disease and an ejection fraction of 55% with poor visualization of the ascending aorta. Figure 2 demonstrates significant CTA findings.
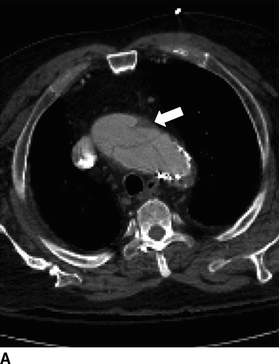
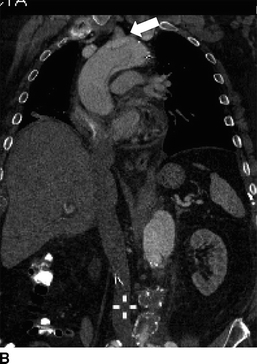
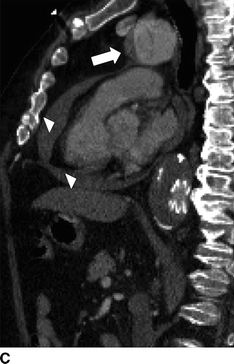
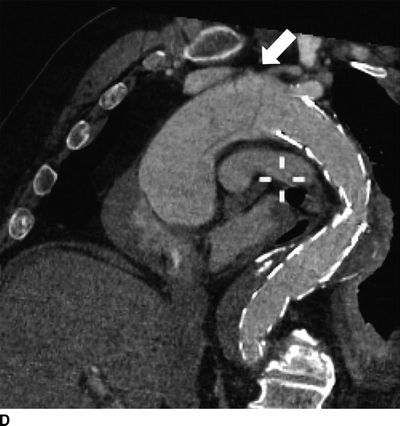
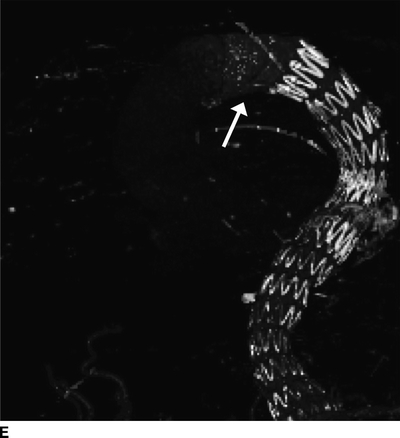
FIGURE 2 Acute type A dissection following TEVAR.CTA demonstrates acute type A aortic dissection (arrows) and pericardial effusion (arrowhead) in axial (A), coronal (B), sagittal (C), and multiplane reconstruction, (D) three-dimensional reconstruction of the aorta demonstrating intimal tear (thin arrow) and aortic endograft (E).
Diagnosis and Treatment
Acute Type A Aortic Dissection
CTA demonstrates a type A aortic dissection extending to the origin of the innominate artery and a moderate pericardial effusion (arrowhead, Fig. 2C). There is no involvement of the arch vessels or evidence of contrast within the false lumen of the previous type B dissection.
The presence of noncovered stent grafts or barbs in the setting of a type B aortic dissection or a thoracic aortic aneurysm may place patients who have undergone TEVAR at increased risk for a retrograde type A dissection. Other risk factors for retrograde dissection following TEVAR include landing zones in the proximal ascending aorta (zone 0) and an ascending aortic diameter greater than 4.0 cm.
Surgical Approach
Treatment for an acute type A aortic dissection is emergent surgical replacement of the involved aortic segment with exclusion of the entry tear. The specific procedure and the need for deep hypothermic circulatory arrest (DHCA) are dictated by the extent of the dissection flap and the site of the entry tear. Dissection may extend down the aortic root leading to aortic insufficiency occasionally requiring valve replacement. Involvement of arch vessels can typically be addressed with a hemiarch replacement; however, an aortic debranching procedure and total arch replacement may rarely be necessary if an arch aneurysm is present, the details of which are beyond the scope of this chapter.
In this patient, the dissection flap appeared to begin at the proximal portion of the existing endograft. Evaluation of the true extent of the dissection flap and whether a complete or partial arch replacement, or an ascending aortic replacement alone must be made intraoperatively, but can be planned based upon the preoperative CTA.
The patient is placed supine with both arms tucked, and a transesophageal echo probe is placed after induction of anesthesia. Exposure of the ascending aorta and arch is achieved through a median sternotomy. The cannulation strategy is aided by the preoperative CTA. Given the involvement of the distal ascending aorta, arterial cannulation is performed through the right axillary artery, the femoral artery, or directly in the ascending aorta proper. In this case, the innominate artery was not dissected, and thus, a right axillary cutdown is performed. Following heparinization, the right axillary artery is clamped and an 8-mm Dacron graft is sewn end to side to the axillary artery to allow for arterial cannulation. A median sternotomy is performed and pericardium opened. In cases of pericardial tamponade, the pericardium is opened gradually to avoid significant hypertension and further injury to the aorta.
Venous cannulation is achieved with a single, multistaged cannula placed in the right atrium. If retrograde cerebral perfusion is planned, a small cannula is placed in the superior vena cava with an umbilical tape placed around the superior vena cava (SVC). Cardioplegia is delivered through a retrograde catheter placed in the coronary sinus. Cardiopulmonary bypass (CPB) is initiated, and the patient is cooled to 18°C to 25°C depending on the planned time for circulatory arrest. The left ventricle is protected from distention with an LV vent placed in the right superior pulmonary vein and across the mitral valve. The appropriate graft size is determined. Once cooling temperature is achieved, the head is packed in ice and the patient’s blood volume is emptied out and circulatory arrest initiated. Retrograde cardioplegia is used to arrest the heart. Antegrade (through the innominate artery with partial arterial flow) or retrograde isolated cerebral perfusion (through the superior vena cava) should be considered when the circulatory arrest period is estimated to exceed 30 minutes.
The mid–ascending aorta is then transected and inspected for the entry tear. In this case, the tear lies near the proximal portion of the previously placed endograft. Thus, an ascending aortic replacement with a hemiarch anastomosis is suitable. The distal ascending aortic graft is anastomosed to the aortic arch at the level of the innominate artery in an end-to-end fashion with a Teflon felt strip to reinforce the dissected aorta. The graft is then clamped and CPB is restarted either through the existing arterial cannula or through an arterial cannula is placed through a side arm of the ascending graft, and rewarming is begun. Significant leaks at this anastomosis should be repaired prior to the proximal anastomosis. Proximally, the graft is cut to an appropriate length. The aorta is transected at the sinotubular junction just above the coronary arteries. If the root is dissected (as is typical), a tailored Teflon felt strip is be placed between the layers and secured in place with Bioglue as a sandwich. The aortic valve is resuspended with three pledgeted sutures at the commissures to ensure aortic valve competence.
The proximal graft is then anastomosed to the repaired aortic root. Ventricular pacing wires are placed, and the cross-clamp is removed. The heart function is assessed as the patient is weaned from CPB. The cannulas are removed as protamine is given. The right axillary conduit is oversewn, and the axillary incision is closed. The side arm of the aortic graft is oversewn. The field is inspected for hemostasis, chest tubes are placed, and the chest is closed (Table 1). This patient underwent this procedure as described without any immediate complications.
TABLE 1. Ascending Aortic Replacement for Type A Dissection




