Tumors and Masses
Detection of a large intracardiac mass is an impressive experience for clinical echocardiographers. Some cardiac masses are suspected from the clinical presentation of the patient and others are incidental findings. Occasionally, a normal structure or a variant of a normal structure may appear as an intracardiac mass. Because many cardiac masses require surgical removal, it is essential to identify correctly any mass visualized in or around the heart. Transesophageal (TEE), intracardiac, and multidimensional echocardiography enhances our ability to diagnose correctly such masses encountered during transthoracic echocardiography (TTE). Cardiac masses can be classified as a cardiac tumor, thrombus, vegetation, iatrogenic material, normal variant, or extracardiac structure. These masses usually can be differentiated by their size, shape, location, mobility, and attachment site as well as by their clinical presentation. Accurate diagnosis is crucial because misinterpretation may lead to an incorrect management strategy, including an unnecessary surgical procedure.
Tumors
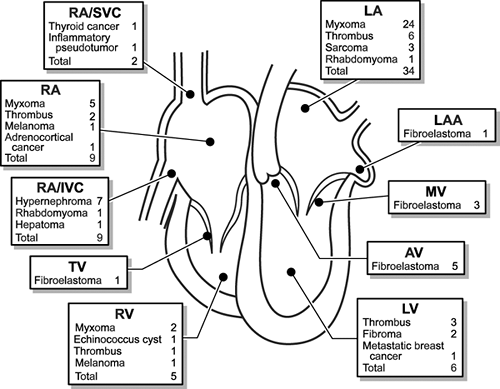
Cardiac tumors are primary, originating from the heart, or they are secondary to metastases from other malignancies. Primary intracardiac tumors occur in at least 7/10,000 people (1). Although primary cardiac tumors usually are benign, they can cause systemic symptoms, embolic events, malignant arrhythmias, chest pain, and heart failure. Because of these potential complications, it is recommended that cardiac tumors be removed whenever possible. The study most frequently cited to describe the spectrum of cardiovascular tumors is that of the Armed Forces Institute of Pathology (2) (Table 18-1). From 1993 to 1998, 75 consecutive patients (34 men with a mean age of 56 ± 16 years) who had cardiac surgery at Mayo Clinic Rochester for removal of a cardiac mass were evaluated with intraoperative TEE (3). The distribution, pathologic type, and attachment sites of the tumors are shown in Table 18-2 and Figure 18-1.
Myxoma
Myxoma is the most common cardiac tumor, accounting for 20% to 30% of intracardiac tumors (1,4). Its most common location is the left atrium (LA), with the attachment site at the atrial septum. It has a typical M-mode and two-dimensional (2D) echocardiographic appearance (Fig. 18-2). Other locations and attachment sites have been observed, including the right atrium (RA), right ventricle (RV), left ventricle (LV), and atrioventricular valve (1,3,4) (Fig. 18-3 and 18-4). In 5% of cases, these tumors are multiple. An atypically located myxoma is usually familial myxoma syndrome in young patients (in combination with lentiginosis and endocrine abnormalities). Carney complex is an autosomal dominant syndrome characterized by cutaneous disease (lentigines, ephelides, and blue nevi) associated with intracardiac myxomas. Myxomas associated with a familial condition are usually located in the atria, but ventricular tumors also occur (5). Familial atrial myxomas account for 7% of all atrial myxomas. Although sporadic atrial myxomas usually are highly amenable to curative surgical resection, familial myxomas frequently recur, often at locations distant from the initial site of resection (6). A defect in a gene on the long arm of chromosome 17 causes Carney complex (5). Atrial myxomas appear gelatinous and friable, with occasional central necrosis. Embolic events are more frequent with a small myxoma (4). The tumor can obstruct the atrioventricular valve (i.e., symptoms similar to those of mitral or tricuspid valve stenosis) and produce a tumor plop that is heard on auscultation after the second heart sound and can be misinterpreted as an opening snap. Ventricular myxoma can obstruct the outflow tract (Fig. 18-3 A), and an outflow murmur is common. Doppler echocardiography is valuable
in detecting the extent of valvular obstruction caused by an atrial myxoma (Fig. 18-2).
in detecting the extent of valvular obstruction caused by an atrial myxoma (Fig. 18-2).
Table 18-1 Relative incidence of tumors of the heart | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Myxoma should be at the top of the list of the differential diagnosis of all intracavitary cardiac tumors. The diagnosis is almost assured if the attachment site to the atrial septum can be established with echocardiography. Rarely, a thrombus can be attached to the atrial septum and even show neovascularization (6). The differential diagnosis of an atrial tumor also includes metastatic tumors (sarcoma and melanoma in the LA and hypernephroma, hepatoma, melanoma, and intravenous leiomyomatosis in the RA) (Fig. 18-5). Atrial tumors can be characterized further by percutaneous transvenous biopsy guided by TTE or TEE, especially if the tumor is in the RA (7).
Fibroma
Fibromas usually are located in the LV free wall, in the ventricular septum, or at the apex. A fibroma is well demarcated from the surrounding myocardium by multiple calcifications (Fig. 18-6). It occasionally grows into the LV cavity and interferes with ventricular filling (Fig. 18-7). Potential problems resulting from a fibroma are congestive heart failure and malignant arrhythmias. When the tumor is located at the apex, the condition may be misinterpreted by other imaging modalities as apical hypertrophic cardiomyopathy. Echocardiography is helpful in diagnosing fibroma and in following the growth of the tumor in asymptomatic patients.
Rhabdomyoma
Rhabdomyoma is the most common cardiac tumor in children, particularly in those with tuberous sclerosis (8). It is often multiple and present in the RV or RV outflow tract and even in the pulmonary artery (Fig. 18-8). The presence of a rhabdomyoma can be diagnosed before birth with fetal echocardiography. This tumor may regress spontaneously after birth.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree