Pericardial Diseases
A normal pericardium consists of an outer sac called the fibrous pericardium and an inner double-layered sac called the serous pericardium. The visceral layer of the serous pericardium, or epicardium, covers the heart and proximal great vessels. It is reflected to form the parietal pericardium, which lines the fibrous pericardium (Fig. 17-1). The pericardium provides mechanical protection for the heart and lubrication to reduce friction between the heart and surrounding structures. The pericardium also has an important hemodynamic effect on the atria and ventricles. The nondistensible pericardium limits acute distention of the heart. Ventricular volume is greater at any given ventricular filling pressure with the pericardium removed than with the pericardium intact. The pericardium also contributes to diastolic coupling between the two ventricles: the distention of one ventricle alters the filling of the other, an effect that is important in the pathophysiology of cardiac tamponade and constrictive pericarditis. Ventricular interdependence becomes more marked at high ventricular filling pressures. Abnormalities of the pericardium can range from the pleuritic chest pain of pericarditis to marked heart failure and even death from tamponade or constriction.
Echocardiography is the most important clinical tool in the diagnosis and management of various pericardial diseases. Pericardial effusion, tamponade, pericardial cyst, and the absence of pericardium are readily recognized on two-dimensional (2D) echocardiography. The detection of pericardial effusion was clinically very difficult before the advent of echocardiography, and it was one of the most exciting initial clinical applications of cardiac ultrasonography more than 40 years ago (1). When a pericardial effusion needs to be drained, pericardiocentesis is performed most safely under the guidance of 2D echocardiography (2). Although it may be difficult to establish the diagnosis of constrictive pericarditis with 2D echocardiography alone, the characteristic respiratory variation in mitral inflow and hepatic vein Doppler velocities and tissue Doppler recording of the mitral anulus velocity have added reliability and confidence to the noninvasive diagnosis of constrictive pericarditis (3,4,5). Transesophageal echocardiography (TEE) is helpful in measuring pericardial thickness (6), in evaluating diastolic function (for tamponade or constrictive physiology) from the pulmonary vein, and in detecting loculated pericardial effusion or other structural abnormalities of the pericardium. The various applications of echocardiography in the evaluation of pericardial diseases are illustrated in this chapter.
Congenitally Absent Pericardium
The congenital absence of the pericardium usually involves the left side of the pericardium. Complete absence of the pericardium on the right side is uncommon. The defect is more frequent in males. It rarely causes symptoms such as chest pain, dyspnea, or syncope. Because of
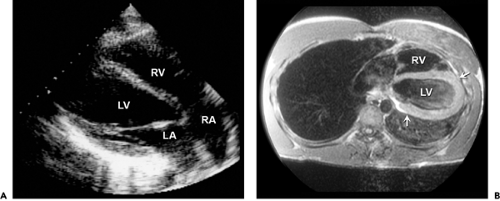
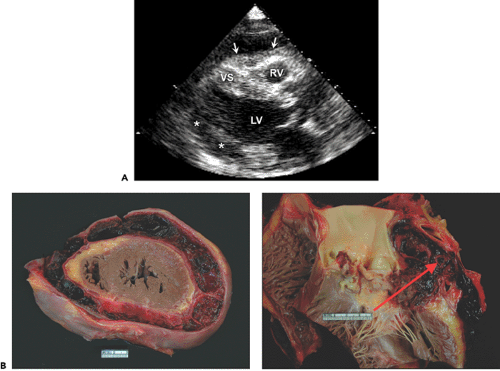
the pericardial defect, cardiac motion is exaggerated, especially the posterior wall of the left ventricle (LV). The entire cardiac structure is shifted to the left; hence, the right ventricular (RV) cavity appears enlarged from the standard parasternal windows, mimicking the RV volume overload pattern on echocardiography (7,8). The absence of pericardium should be considered when the RV appears enlarged from the parasternal window and is at the center of the usual apical image (Fig. 17-2 A). This condition is also associated with a high incidence of atrial septal defect, bicuspid aortic valve, and bronchogenic cysts. It is readily recognized because of its typical 2D echocardiographic features. The diagnosis can be confirmed with computed tomography or magnetic resonance imaging (Fig. 17-2 B).
the pericardial defect, cardiac motion is exaggerated, especially the posterior wall of the left ventricle (LV). The entire cardiac structure is shifted to the left; hence, the right ventricular (RV) cavity appears enlarged from the standard parasternal windows, mimicking the RV volume overload pattern on echocardiography (7,8). The absence of pericardium should be considered when the RV appears enlarged from the parasternal window and is at the center of the usual apical image (Fig. 17-2 A). This condition is also associated with a high incidence of atrial septal defect, bicuspid aortic valve, and bronchogenic cysts. It is readily recognized because of its typical 2D echocardiographic features. The diagnosis can be confirmed with computed tomography or magnetic resonance imaging (Fig. 17-2 B).
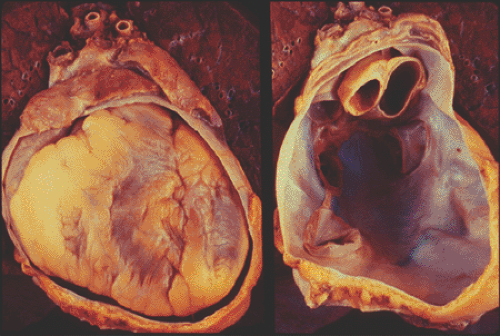 Figure 17-1 Pathology specimens showing the double-layered pericardium with (left) and without (right) the heart in the fibrous pericardial cavity. (Courtesy W. D. Edwards, MD.) |
Pericardial Cyst
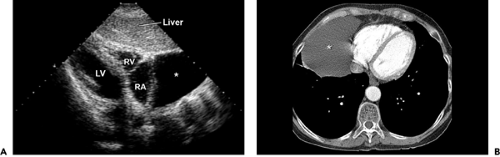
A pericardial cyst is typically a benign structural abnormality of the pericardium that usually is detected as an incidental mass lesion on chest radiography or as a cystic mass on echocardiography (Fig. 17-3 A). Most frequently,
pericardial cysts are located in the right costophrenic angle, but they also are found in the left costophrenic angle, hilum, and superior mediastinum. Pericardial cysts need to be differentiated from malignant tumors, cardiac chamber enlargement, and diaphragmatic hernia. Two-dimensional echocardiography is useful in differentiating a pericardial cyst from other solid structures because a cyst is filled with clear fluid and appears as an echo-free structure. It also has a characteristic appearance on computed tomography and magnetic resonance imaging (Fig. 17-3 B).
pericardial cysts are located in the right costophrenic angle, but they also are found in the left costophrenic angle, hilum, and superior mediastinum. Pericardial cysts need to be differentiated from malignant tumors, cardiac chamber enlargement, and diaphragmatic hernia. Two-dimensional echocardiography is useful in differentiating a pericardial cyst from other solid structures because a cyst is filled with clear fluid and appears as an echo-free structure. It also has a characteristic appearance on computed tomography and magnetic resonance imaging (Fig. 17-3 B).
Pericardial Effusion and Tamponade
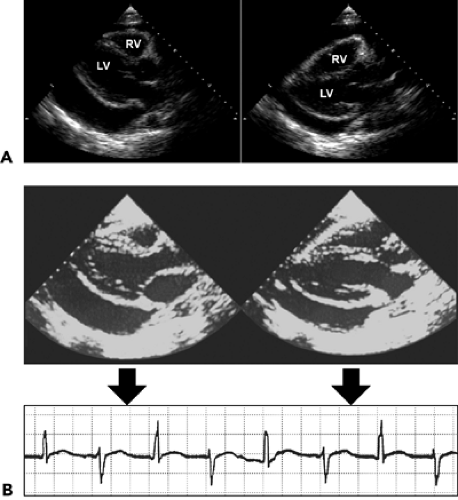
Filling of the potential pericardial space with fluid or blood results in a pericardial effusion, which is detected as an echo-free space. When the amount of effusion is more than 25 mL, an echo-free space persists throughout the cardiac cycle. A smaller amount of pericardial effusion may be detected as a posterior echo-free space that is present only during the systolic phase. As the pericardial effusion increases, movement of the parietal pericardium decreases. When the amount of pericardial effusion is massive, the heart may have a “swinging” motion in the pericardial cavity (Fig. 17-4 A), which is responsible for the electrocardiographic (ECG) manifestation of cardiac tamponade called electrical alternans (Fig. 17-4 B). However, the swinging motion is not always present in cardiac tamponade. Cardiac tamponade can occur with a small amount of pericardial effusion if the effusion accumulates rapidly (9). Tamponade occurs when the intrapericardial pressure increases to the point of compromising systemic venous return to the right atrium (RA). Increased intrapericardial pressure reduces the myocardial transmural pressure (= intracardiac pressure – intrapericardial pressure).
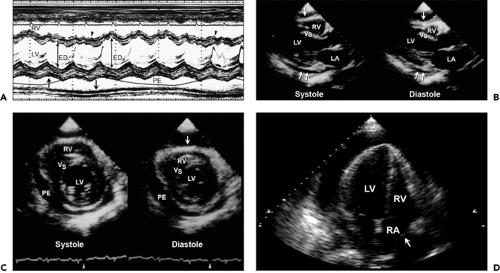
The intrapericardial pressure and volume relationship is much stiffer when the pericardial fluid accumulates rapidly (Fig. 17-5). A clinical example is myocardial perforation after acute myocardial infarction or during placement of a pacemaker lead. Various M-mode and 2D echocardiographic signs have been reported in this life-threatening condition (10,11), including early diastolic collapse of the RV, late diastolic RA inversion, abnormal ventricular septal motion, respiratory variation in ventricle chamber size (Fig. 17-6), and plethora of the inferior vena cava with blunted respiratory changes. These findings are caused by the characteristic hemodynamics of tamponade. Diastolic collapse of the RA and RV are related to intrapericardial pressure rising above the intracardiac pressures, and abnormal ventricular septal motion is related to respiratory variation in ventricular filling. Diastolic collapse of the right heart may not occur if right-heart pressure is elevated. In case of acute myocardial rupture or proximal aortic dissection, clotted blood may be found in the pericardial sac; this finding is highly suggestive of
hemopericardium or coagulum tamponade (Fig. 17-7). When air occurs in the pericardial sac (pneumopericardium) as a result of esophageal perforation, cardiac imaging (both transthoracic echocardiography [TTE] and TEE) is difficult because ultrasound does not penetrate air well.
hemopericardium or coagulum tamponade (Fig. 17-7). When air occurs in the pericardial sac (pneumopericardium) as a result of esophageal perforation, cardiac imaging (both transthoracic echocardiography [TTE] and TEE) is difficult because ultrasound does not penetrate air well.
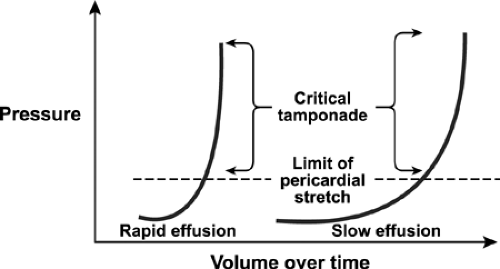 Figure 17-5 Cardiac tamponade. Pericardial pressure-volume (or strain-stress) curves showing volume increases slowly or rapidly over time. Left, Rapidly increasing pericardial fluid first reaches the limit of the pericardial reserve volume (the initial flat segment) and then quickly exceeds the limit of parietal pericardial stretch, causing a steep rise in pressure, which becomes even steeper as smaller increments in fluid cause a disproportionate increase in the pericardial pressure. Right, A slower rate of pericardial filling takes longer to exceed the limit of pericardial stretch because there is more time for the pericardium to stretch and for compensatory mechanisms to be activated. (From Spodick [9]. Used with permission.) |
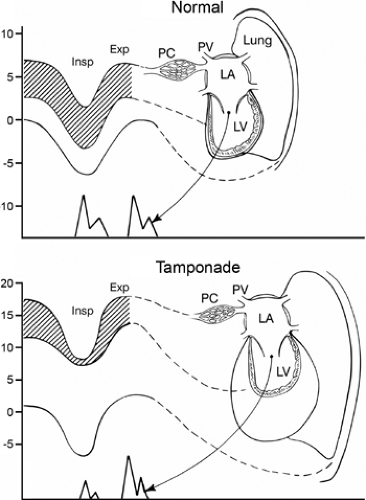
The Doppler echocardiographic features of pericardial effusion and tamponade are more sensitive than the 2D echocardiographic features mentioned above (12,13). The Doppler findings of cardiac tamponade are based on the following characteristic respiratory variations in intrathoracic and intracardiac hemodynamics (Fig. 17-8). Normally, intrapericardial pressure (hence, left atrial [LA] and LV diastolic pressures) and intrathoracic pressure (hence, pulmonary capillary wedge pressure) fall the same degree during inspiration; however, in cardiac tamponade, intrapericardial (and intracardiac) pressure falls substantially less than intrathoracic pressure. Therefore, the LV filling pressure gradient (from pulmonary capillary wedge pressure to LV diastolic pressure [shaded area in Fig. 17-8]) decreases with inspiration. Consequently, mitral valve opening is delayed, which lengthens the isovolumic relaxation time (IVRT) and decreases mitral E velocity. In cardiac tamponade, the degree of ventricular filling depends on the other ventricle because of the relatively fixed combined cardiac volume (ventricular interdependence); thus, reciprocal changes occur in the right-heart chambers (Fig. 17-9). Increased venous return to the right-heart chambers with inspiration also contributes to the increased ventricular interdependence.
The changes in respiratory flow velocity across the mitral and tricuspid valves are also reflected in the pulmonary and hepatic venous flow velocities, respectively: an inspiratory decrease and an expiratory increase in pulmonary vein diastolic forward flow and expiratory decrease in hepatic vein forward flow and increase in expiratory reversal flow (Fig. 17-10). In an urgent
situation, Doppler echocardiography is performed without a simultaneous respirometer recording (Fig. 17-11). Typical mitral inflow and hepatic vein Doppler velocity changes need to be demonstrated to diagnose hemodynamic compromise due to pericardial effusion. Hepatic vein forward flow velocity is always reduced with expiration (unless patient is mechanically ventilated), at which time diastolic reversal flow is greatest if there is tamponade or constriction.
situation, Doppler echocardiography is performed without a simultaneous respirometer recording (Fig. 17-11). Typical mitral inflow and hepatic vein Doppler velocity changes need to be demonstrated to diagnose hemodynamic compromise due to pericardial effusion. Hepatic vein forward flow velocity is always reduced with expiration (unless patient is mechanically ventilated), at which time diastolic reversal flow is greatest if there is tamponade or constriction.
Echocardiographically Guided Pericardiocentesis
The most effective treatment for cardiac tamponade is removal of the pericardial fluid. Although pericardiocentesis is lifesaving, a blind percutaneous attempt has a high rate of complications, including pneumothorax, puncture of the cardiac wall, and death. Two-dimensional echocardiography can guide pericardiocentesis by locating the optimal site of puncture (Fig. 17-12), by determining the depth of the pericardial effusion and the distance from the puncture site to the effusion, and by monitoring the results of the pericardiocentesis, usually from the subcostal view. The position of the pericardiocentesis needle can be confirmed by imaging with administration of agitated saline. Figure 17-13 shows contrast in the pericardial space, not in the RV. Under most circumstances, a pigtail (6F or 7F) catheter is introduced and left in the pericardial sac for several days, with intermittent drainage (every 4–6 hours); this has markedly curtailed the rate of recurrent effusion and the use of a sclerosing agent. At Mayo Clinic, most pericardiocentesis procedures are performed by an echocardiographer with the guidance of 2D echocardiography (2). The most
common location for entry of the needle is the parasternal or apical area; the subxiphoid location is used less frequently, depending on the 2D echocardiographic findings. In 1,127 consecutive echocardiographically guided pericardiocentesis procedures in our laboratory, malignant effusion was the most common reason for the procedure (34% of cases), followed by a postoperative complication (25% of cases), complication of a catheter-based procedure (10% of cases), and others (Fig. 17-14). The procedure was successful in 97% of patients. Complications were mostly minor; major complications included death (1 patient), cardiac laceration (5), vessel laceration (1), pneumothorax (5), infection (1), and sustained ventricular tachycardia (1).
common location for entry of the needle is the parasternal or apical area; the subxiphoid location is used less frequently, depending on the 2D echocardiographic findings. In 1,127 consecutive echocardiographically guided pericardiocentesis procedures in our laboratory, malignant effusion was the most common reason for the procedure (34% of cases), followed by a postoperative complication (25% of cases), complication of a catheter-based procedure (10% of cases), and others (Fig. 17-14). The procedure was successful in 97% of patients. Complications were mostly minor; major complications included death (1 patient), cardiac laceration (5), vessel laceration (1), pneumothorax (5), infection (1), and sustained ventricular tachycardia (1).
Pericardial Effusion Versus Pleural Effusion
Pericardial effusion usually is located circumferentially. If the echo-free space is found only anteriorly, the condition is more likely to be an epicardial fat pad than a pericardial effusion. Posteriorly, a pericardial effusion is anterior to the descending thoracic aorta, whereas a pleural effusion is posterior to the aorta (Fig. 17-15). Two-dimensional ultrasonographic imaging of a pleural effusion is also helpful in thoracentesis to locate the optimal puncture site. A pleural effusion on the left side allows cardiac imaging from the back (Fig. 17-16).
Constrictive Pericarditis
Constrictive pericarditis is caused by a thickened, inflamed, adherent, or calcific pericardium that limits diastolic filling of the heart (Fig. 17-17). Constrictive pericarditis is not an uncommon condition, but it frequently escapes clinical detection. It is not considered in many cases because the clinical presentation mimics that of other more common diseases and no one diagnostic test alone can ensure, with high confidence, the diagnosis of constrictive pericarditis. Because constrictive pericarditis is a curable cause of severe heart failure, it should be considered in all patients who have heart failure, especially if systolic function is normal or there is a predisposing factor. Currently, the most common cause of constriction is previous cardiac surgery, followed by pericarditis, an episode of pericardial effusion, and radiotherapy (14,15). The underlying causes of constriction in more than 400 patients who have had pericardiectomy since 1985 at Mayo Clinic are listed in Figure 17-18. Patients with constrictive pericarditis present with dyspnea, peripheral edema, ascites, pleural effusion, fatigue, or anasarca. The jugular venous pressure is almost always elevated, with a typical rapid “y” descent (Fig. 17-19). Other typical physical findings are the Kussmaul sign and pericardial knock. Because of abdominal symptoms and elevation of liver enzymes from hepatic venous congestion, many patients are thought to have liver or gastrointestinal disease and undergo noncardiac procedures such as liver biopsy, endoscopy, and even abdominal exploration before constrictive pericarditis is diagnosed.
Pericardial calcification seen on chest radiography is helpful but occurs in only 23% of patients (16) (Fig. 17-20). Although a thick pericardium is a usual finding in this condition, pericardial thickness may be normal in up to 20% of patients (17). Traditional invasive hemodynamic features do not overlap much with those of restrictive cardiomyopathy or other myocardial diseases. B-type natriuretic peptide levels tend to be lower (or even normal) in constrictive pericarditis than in restrictive cardiomyopathy (18). New insights into
the mechanism of constrictive pericarditis have allowed the development of more reliable and specific diagnostic criteria, using comprehensive echocardiography with 2D, Doppler, and tissue Doppler imaging (3,4,5,19



the mechanism of constrictive pericarditis have allowed the development of more reliable and specific diagnostic criteria, using comprehensive echocardiography with 2D, Doppler, and tissue Doppler imaging (3,4,5,19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree