Truncus Arteriosus
Michael O. Murphy
Thomas L. Spray
Truncus arteriosus represents an unusual congenital heart defect in which a single arterial trunk arises from the heart, providing the origins of the coronary arteries, the true pulmonary arteries, and the brachiocephalic vessels. The defect is usually associated with a ventricular septal defect and a single, large semilunar valve. Typically, the semilunar valve contains up to four separate leaflets, which may be dysmorphic in infancy. In situations in which more than four leaflets are present, incorporation of remnants of the pulmonary valve into the truncal valve is presumed. Very rare instances of absence of ventricular septal defect in truncus arteriosus have been reported, but usually the infundibular septum is virtually absent superiorly. Thus, the ventricular septal defect is similar to that seen in tetralogy of Fallot but with no superior rim of infundibular septum separating the pulmonary and aortic valves. This feature can be used to distinguish truncus arteriosus from pulmonary atresia with ventricular septal defect. Embryologically, the development of truncus arteriosus is related to deficiency of the aorticopulmonary septum, with the absence of the subpulmonary infundibulum and partial or complete absence of pulmonary valve tissue. These defects are associated with neural crest abnormalities, and because the neural crest also develops into thymus and parathyroids, DiGeorge syndrome is commonly associated with truncus arteriosus.
The development of aortic arches 4 and 6 varies in truncus arteriosus such that infants with hypoplasia or interruption of the aortic arch have associated large ductal connections between the truncus and the descending aorta, and infants in whom the arch is fully developed usually have absence of the ductus arteriosus.
The primary classification systems for truncus arteriosus have focused on the origins of the pulmonary arteries. The classification scheme of Collett and Edwards defines truncus arteriosus types by the presence of a main pulmonary trunk or the separation of the pulmonary arteries from the arterial trunk. From a surgical standpoint, however, the variations in the origin of the pulmonary arteries are often similar enough among the various types that separation by this classification scheme has not been as useful as the alternate scheme developed by Van Praagh. The Van Praagh classification system is described in Table 87.1. Most recently, a modified Van Praagh classification has been proposed by the Congenital Heart Surgery Nomenclature and Database project, classifying cases by the presence of confluent, or near confluent pulmonary arteries, truncus arteriosus with the absence of one pulmonary artery or truncus arteriosus in the presence of interrupted aortic arch or coarctation. The advantage of this system is that it is based on a hierarchical system and can be used interchangeably with the traditional classification systems.
Some authors have described “hemitruncus” as a situation in which the right pulmonary artery arises from the ascending aorta and the left pulmonary artery from the right ventricle. This defect is not typically associated with a ventricular septal defect. In our opinion, it is not a variation of truncus arteriosus and is best defined as aortic origin of the right pulmonary artery. Such a description distinguishes this anomaly from the Van Praagh type 3A truncus.
The truncal valve typically straddles the ventricular septum with balanced contributions from the left and right ventricles. However, in a few cases the truncal valve straddles the right ventricle, offering the potential for restriction of blood flow from the left ventricle to the truncal valve at repair. Coronary anomalies seen in truncus are typically a high origin of the left coronary artery from the truncus near the pulmonary origins and an anomalous anterior descending coronary from the right coronary artery crossing the right ventricle to supply the anterior ventricular septum.
PATHOPHYSIOLOGY OF TRUNCUS ARTERIOSUS
The defect of truncus arteriosus in the absence of arch interruption or severe hypoplasia is one of complete mixing of systemic and pulmonary venous blood with pulmonary overcirculation. As the pulmonary vascular resistance falls in the early neonatal period, the tendency is for unrestricted pulmonary blood flow from the truncus, resulting in severe congestive heart failure. Mild cyanosis may be present because of the complete mixing at the level of the atrium and ventricle. The presence of a large connection between the ascending aorta and the pulmonary arteries above the level of the semilunar valve results in pulmonary blood flow during systole and diastole, increasing the amount of pulmonary overcirculation. It is believed that this situation results in early development of pulmonary vascular obstructive disease in children with truncus arteriosus, which accounts for the high mortality of untreated patients with this condition. Infants born with truncus arteriosus and unrestricted pulmonary blood flow have a mortality of as high as 50% in the first month of life because of congestive heart failure. Pulmonary vascular obstructive disease typically develops to a significant degree by 6 months of age. Other physiologic consequences of truncus arteriosus relate to the presence or absence of truncal valve stenosis or insufficiency. Although major truncal valve stenosis is rare, truncal valve insufficiency occurs in a significant proportion of children with this condition. The presence of significant truncal valve insufficiency not only complicates the operative repair of the lesion but also may have a significant impact on morbidity and mortality after complete repair.
The presence of interrupted aortic arch in association with truncus arteriosus results in cardiovascular instability early after birth. Maintenance of ductal patency by prostaglandin E1 infusion is critical to provide distal perfusion to the body in this condition but is associated with
progressive pulmonary overcirculation, which is not dissimilar to the situation seen in hypoplastic left heart syndrome.
progressive pulmonary overcirculation, which is not dissimilar to the situation seen in hypoplastic left heart syndrome.
Table 87.1 Van Praagh Classification of Truncus Arteriosus | |||
|---|---|---|---|
|
DIAGNOSIS AND INDICATIONS FOR SURGICAL INTERVENTION
The diagnosis of truncus arteriosus can generally be made with accuracy from echocardiograms. Cardiac catheterization is indicated only when the patient presents late after birth and the issue of pulmonary vascular resistance is raised. Some children may have significant pulmonary arterial stenosis, which permits control of congestive heart failure early in life, and catheterization to define the level and degree of obstruction may be beneficial. In addition, abnormalities of coronary arteries and the severity of truncal valve abnormalities may occasionally prompt catheterization for greater characterization of the defects.
We have generally considered the presence of truncus arteriosus an indication for operation in a relatively urgent fashion. Because hemodynamics may be quite unstable in children with unrestricted pulmonary blood flow and because we have seen patients deteriorate rapidly while awaiting surgical intervention, we have elected to repair truncus arteriosus promptly after the diagnosis is made. This policy reflects the clear advantages of neonatal repair of truncus arteriosus. Review of series of truncus repairs reflects the trend toward earlier intervention. Whereas the first repairs of truncus arteriosus were performed in 1962 by Behrendt and associates, in 1967 McGoon first used a valved allograft for the repair. This operation was a refinement of the procedure described by Rastelli at the Mayo Clinic. The results of early repairs were quite poor, however, because of the development of pulmonary vascular obstructive disease. In most of these early repairs, the operation was undertaken after the patient reached 6 months of age, when pulmonary vascular resistance was already significantly elevated. In 1984, Ebert reported a series of 100 infants who underwent complete repair of truncus arteriosus at 6 months of age with an 11% mortality. This landmark report emphasized the improved results in early intervention in children with this lesion. In our experience, repair of truncus arteriosus has gradually improved as surgical techniques have evolved such that operative survivals of >95% are anticipated with neonatal repair in patients without associated valvar lesions. The late results of truncus arteriosus have been favorable, with little late mortality despite the need for reoperation to replace right ventricular outflow tract conduits.
 SURGICAL TECHNIQUE
SURGICAL TECHNIQUERepair of Truncus Arteriosus
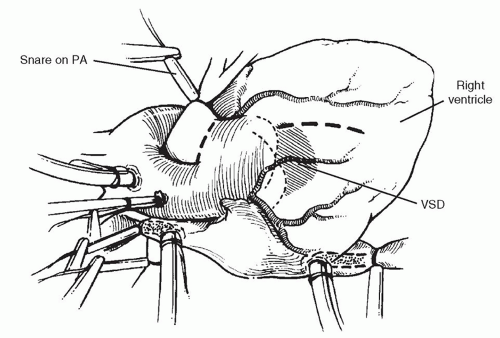
The heart is exposed with a median sternotomy incision. The presence of thymic tissue is confirmed because many of these children have athymia associated with DiGeorge syndrome. The clear presence of thymic tissue, therefore, may have implications for later management and prognosis. The heart is suspended in a pericardial cradle and the great vessels examined. If the patient is severely overcirculated with congestive heart failure, it may be advisable to promptly encircle one of the pulmonary arteries with a snare to limit the total pulmonary blood flow. On occasion, this can stabilize the patient while dissection is performed around the arch vessels.
The operation can be performed either with the patient on continuous cardiopulmonary bypass or by utilizing circulatory arrest. We have elected to use both of these techniques, and in simple truncus arteriosus, the complete operative repair can be performed during a single period of circulatory arrest of <40 minutes in most cases. The right and left pulmonary arteries are mobilized and encircled with tourniquets for control and the origins of the pulmonary arteries examined. In addition, on opening the pericardium, it is important to determine the location of the anterior descending coronary artery and confirm that it does not cross the right ventricular wall in the area of anticipated ventriculotomy. Heparin is administered and the aorta cannulated as distally as possible. Distal cannulation is particularly important because the pulmonary bifurcation may come off relatively distally and there is little room for cross-clamping of the aorta between the anticipated division of the pulmonary bifurcation and the distal aorta. Either the venae cavae are cannulated if continuous bypass is used, or a single right atrial cannula is used with circulatory arrest. If mobilization of the pulmonary artery confluence behind the aorta is considered to be too close to the anticipated cross-clamp application site, the brachiocephalic vessels are encircled with tourniquets and circulatory arrest is used, with clamping of the aorta more distally on the arch during the reconstruction of the back of the truncus arteriosus after excision of the pulmonary bifurcation. The infant is placed on cardiopulmonary bypass and the snares on the right and left pulmonary arteries tightened as shown in Figure 87.1 to prevent pulmonary overcirculation during cooling. The child is cooled to 18°C for circulatory arrest or 34 to 37°C for continuous bypass repair. In the presence of significant truncal valve insufficiency, venting of the left ventricle is important during cooling and may be accomplished by placing a vent through the right superior pulmonary vein across the mitral valve into the ventricle, or if truncal insufficiency is severe, by compressing the heart to maintain emptying of the ventricle during cooling.
The aorta is then cross-clamped and cardioplegic solution is administered into the truncal root with the pulmonary artery snares applied to force the cardioplegic solution into the coronary arteries. Alternatively, cardioplegia can be induced in a retrograde manner or the aorta opened and the coronaries directly cannulated. After cardioplegia is achieved, the snares are removed from the pulmonary arteries, and an incision is made near the takeoff of the pulmonary bifurcation anteriorly. Through this initial incision, the truncal valve is examined and the origin of the left coronary artery carefully identified. The left coronary can arise high from above the truncal sinuses and be intimately associated with the origin of the pulmonary bifurcation. Once the coronary arteries are identified, the pulmonary bifurcation is excised from the back of the truncus arteriosus. The aorta is then repaired using a
patch of pulmonary homograft. Primary suture closure of the defect in the truncus may be used; however, we believe that the placement of a patch allows for less tension on the anastomosis and therefore a decreased risk of bleeding from behind the aorta. This is important after the right ventricular reconstruction has been performed because exposure of the back of the aorta is difficult after the completion of the repair. In addition, suturing bleeding sites in this area may be hazardous because the origin of the left coronary artery may be very close to the margins of the excision of the pulmonary bifurcation. Although pericardium can also be used for a patch, we have elected to use a portion of the pulmonary or aortic homograft that will be used for right ventricular outflow tract reconstruction because it results in a hemostatic suture line and is easier to work with than nontanned pericardium.
patch of pulmonary homograft. Primary suture closure of the defect in the truncus may be used; however, we believe that the placement of a patch allows for less tension on the anastomosis and therefore a decreased risk of bleeding from behind the aorta. This is important after the right ventricular reconstruction has been performed because exposure of the back of the aorta is difficult after the completion of the repair. In addition, suturing bleeding sites in this area may be hazardous because the origin of the left coronary artery may be very close to the margins of the excision of the pulmonary bifurcation. Although pericardium can also be used for a patch, we have elected to use a portion of the pulmonary or aortic homograft that will be used for right ventricular outflow tract reconstruction because it results in a hemostatic suture line and is easier to work with than nontanned pericardium.
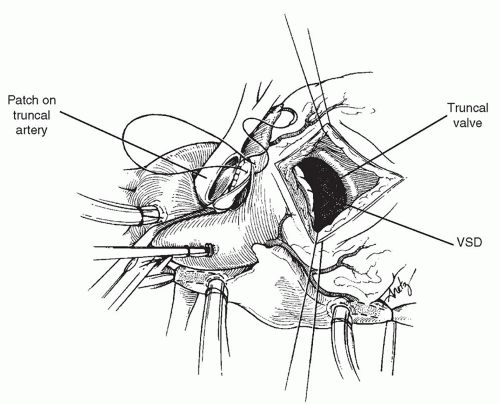
After the repair of the truncus arteriosus is complete and the pulmonary bifurcation is mobilized to bring the bifurcation to the left for reconstruction, additional cardioplegic solution is injected into the aortic root to test the suture line of the truncal patch, and additional sutures are placed if necessary. Next, an incision is made in the right ventricle, avoiding major conal branches of the coronary arteries. This incision is made at the base of the truncal valve, and care must be taken not to carry the incision too far superiorly into the truncal valve annulus. The absence of infundibular septum in this area results in the possibility of damage to the truncal valve if the incision is started too far superiorly. The orientation of the incision is made so that the takeoff of the conduit from the right ventricle will be directed toward the pulmonary bifurcation.
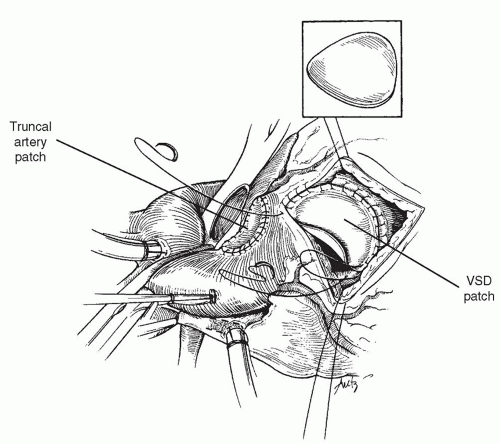
As shown in Figure 87.2, the ventricular septal defect is exposed through the incision in the right ventricle. The defect is closed with a patch of polyester (Dacron) material using a running technique. Superiorly, the absence of the infundibular septum mandates that the patch be more ovoid in shape, and the patch is secured to the epicardial margin of the ventriculotomy incision superiorly to avoid interference with the truncal valve (Fig. 87.3). In the majority of cases, there is a bridge of muscle between the margin of the ventricular septal defect and the tricuspid valve septal leaflet, and in these patients, suturing in this area can be performed without risk to the conduction
tissue. In a minority of cases, there is absence of this muscle tissue, and suturing of the ventricular septal patch to the base of the septal leaflet of the tricuspid valve is necessary.
tissue. In a minority of cases, there is absence of this muscle tissue, and suturing of the ventricular septal patch to the base of the septal leaflet of the tricuspid valve is necessary.
After closure of the ventricular septal defect is complete, the right ventricular outflow tract is reconstructed using a pulmonary or aortic homograft. Although in the past we elected to use larger pulmonary homografts to allow for the maximum possible growth of the infant before conduit change is necessary, more recently we have used aortic allografts preferentially, because the high pulmonary vascular resistance seen in the early postoperative period often causes dilation in the pulmonary allografts. Another advantage for the initial use of an aortic homograft is the curved nature of the ascending aorta that can be positioned to the left, curving around the enlarged truncal root in patients in whom the truncus is particularly enlarged.
The use of an aortic homograft in the initial repair of truncus arteriosus prevents pulmonary homograft dilation early. At the time of the conduit replacement after truncus repair which typically occurs between 3 and 6 years after the operation, a pulmonary homograft is preferentially selected since pulmonary vascular resistance is now low and placement of a large pulmonary homograft may obviate the need for additional conduit replacements in the future, and the development of pulmonary insufficiency in the homograft conduit can be treated later with a stent mounted on pulmonary valve with a percutaneous approach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree