Persistent truncus arteriosus is a rare congenital heart defect in which the aortic arch, pulmonary arteries, and coronary arteries arise from a common great artery originating from the base of the heart. It occurs in 1% to 4% of all cases of congenital heart disease. Truncus arteriosus is typically fatal without intervention in early infancy; however, with current surgical therapy most children with truncus arteriosus survive. Most cases are sent to surgery based on echocardiographic imaging alone. Accurate noninvasive imaging is critical to defining the cardiac anatomy, guiding appropriate surgical management, and following these patients in the long term.
DEVELOPMENT AND ANATOMY
Truncus arteriosus occurs within the first 3 to 4 weeks of fetal life when there is failure of the aorticopulmonary septum to form and spiral within the embryonic truncus arteriosus, preventing a partition between the two great arteries. The anatomic description of truncus arteriosus consists of an outlet ventricular septal defect (VSD), a single semilunar valve, and a common great artery that overrides the VSD. Collett and Edwards first established four different anatomic classifications of truncus arteriosus based on the origin of the pulmonary arteries (Fig. 18.1). In type I, the main pulmonary artery arises from the truncal root and bifurcates into a right and a left pulmonary artery. In type II, each pulmonary artery originates from a separate origin off the posterior aspect of the truncal root. In type III, each pulmonary artery arises independently from the lateral aspects of the truncal root. In type IV, no true pulmonary arteries are present and pulmonary blood flow is supplied via aortopulmonary collateral vessels. This type of truncus arteriosus is considered to be a form of pulmonary atresia with VSD. Van Praagh later developed a classification based on truncus patients with a VSD (group A) and those without (group B [very rare]). Among both groups A and B, the four subgroups are the same (see Fig. 18.1). In the Van Praagh classification system, type III truncus arteriosus includes patients with origin of one pulmonary artery from the truncus. In this subset of patients, the other pulmonary artery is supplied via the ductus arteriosus or an aortopulmonary collateral vessel. In the Van Praagh classification system, type IV truncus arteriosus includes interruption of the aortic arch, with the descending aorta supplied by the patent ductus arteriosus. In cases of aortic arch interruption, type B is the most common form of interruption. A modified version of the Collett and Edwards classification was established by Konstantinov and colleagues. Figure 18.2 represents variations of truncus arteriosus with interrupted aortic arch based on a multi-institutional study of 50 neonates in an effort to better define the morphology and characteristics of this particular anomaly. A simplified classification based on pulmonary or aortic dominance has more recently been advocated (Fig. 18.3). This simplified approach emphasizes the principal morphologic determinant of surgical outcome, with patients in the more common aortic dominant category (Fig. 18.3B) having higher survival than the less common pulmonary dominant group (Fig. 18.3A).
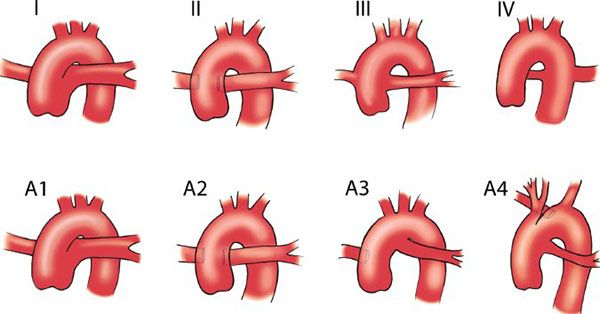
Figure 18.1. Truncus arteriosus classifications. (From Frank L, Dillman JR, Parish V, et al. Cardiovascular MR imaging of conotruncal anomalies. Radiographics. 2010 Jul–Aug;30(4):1069–1094.)
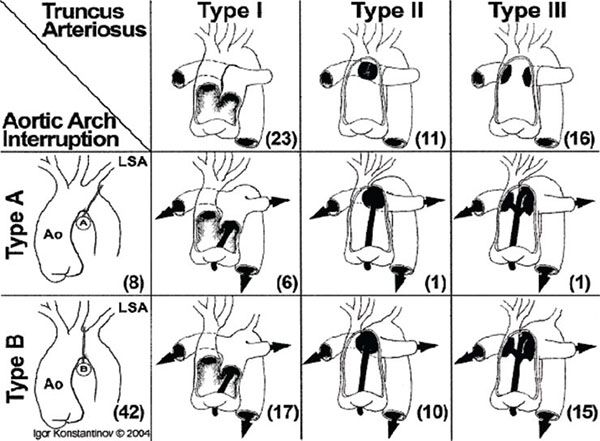
Figure 18.2. Truncus arteriosus with interrupted aortic arch classification. Ao, aorta, LSA, left subclavian artery. (Reproduced with permission from Konstantinov IE, et al. Ann Thorac Surg. 2006;81:214–223.)
The truncal valve commonly overrides both ventricles, but may be more committed to one ventricle than the other. Isolated truncal root origin from a ventricle is more common from the right ventricle than from the left ventricle. The truncal valve has a variable number of leaflets (one to six), and they are often morphologically thickened and dysplastic. Approximately two-thirds of all truncal valves are trileaflet, whereas almost one-quarter are quadricuspid. Less than 10% are found to be bicuspid. The truncal valve may be competent, but is more commonly regurgitant and/or stenotic because of the valve’s abnormal morphology.
CLINICAL PRESENTATION
Truncus arteriosus may be diagnosed prenatally with fetal echocardiography, although it can be difficult to diagnose from traditional obstetrical screening ultrasound four-chamber views. One of the keys to fetal diagnosis of truncus arteriosus is correct identification of the origin of the pulmonary arteries from the posterior aspect of the aorta (Videos 18.1 and 18.2). Patients not diagnosed prenatally with truncus arteriosus typically present during early infancy with tachypnea, feeding difficulty, failure to thrive, or other symptoms and signs related to pulmonary overcirculation as pulmonary vascular resistance falls. Patients with truncus and interrupted aortic arch typically present earlier because of decreased perfusion. The cardiac examination in patients with truncus arteriosus typically demonstrates a hyperactive precordium and a widened pulse pressure. There is characteristically a loud single S2, with a loud systolic murmur. Patients often have a click from the truncal valve and may have a diastolic murmur if there is significant truncal valve regurgitation. The chest radiograph demonstrates cardiomegaly and increased pulmonary vascularity because of an increase in pulmonary blood flow. One-third of patients have a right-sided aortic arch. The electrocardiogram typically shows biventricular hypertrophy.
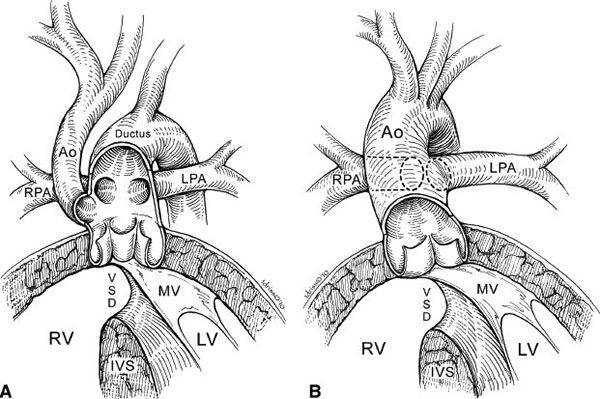
Figure 18.3. Truncus arteriosus: pulmonary versus aortic dominance. Ao, aorta; LPA, left pulmonary artery; LV, left ventricle; MV, mitral valve; RV, right ventricle; RPA right pulmonary artery; VSD, ventricular septal defect. (Reproduced with permission from Russell HM, et al. J Thorac Cardiovasc Surg 2011;141:645–53.)
ASSOCIATED CONDITIONS
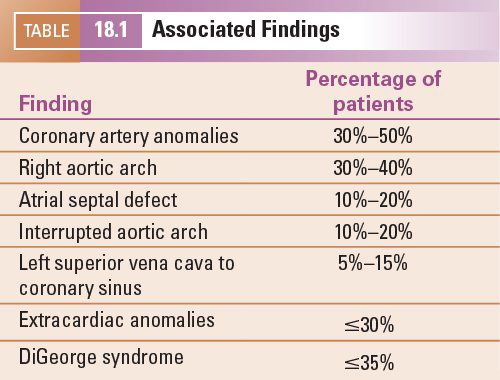
Cardiac abnormalities commonly associated with truncus arteriosus include (a) coronary artery anomalies, with a single coronary artery and an intramural course having the most important surgical implications, (b) right aortic arch, (c) secundum atrial septal defect, (d) left superior vena cava to coronary sinus, and (e) interrupted aortic arch (Table 18.1). Absence or atresia of the ductus arteriosus is usually expected with truncus arteriosus, the exception being truncus arteriosus with an interrupted aortic arch. Extracardiac anomalies include renal, skeletal, intestinal, and systemic defects. The association of truncus arteriosus with DiGeorge syndrome and chromosome 22 deletion is well recognized, with up to 35% of patients with truncus arteriosus having DiGeorge syndrome.
ECHOCARDIOGRAPHIC FEATURES
Two-Dimensional Echocardiographic Examination
Echocardiography has evolved as the modality of choice for diagnosing the many anatomic variations of truncus arteriosus. The anatomic information obtained from two-dimensional and Doppler echocardiography is usually sufficient to send the patient for surgical repair without the need for further testing.
In the parasternal long-axis view, the diagnosis of truncus arteriosus is suggested by a dilated single great artery, in continuity with the mitral valve, arising from the base of the heart, overriding the interventricular septum concomitant with a malalignment type VSD (Fig. 18.4). In truncus arteriosus type I, the short main pulmonary artery segment can frequently be seen arising from the truncal vessel posteriorly and leftward. (Videos 18.3 through 18.7) In some cases of truncus arteriosus, sweeping from the right to the patient’s left in the long axis demonstrates the deficiency of a separate pulmonary valve and the pulmonary arteries arising from the truncal vessel.
The parasternal short-axis view at the base of the heart is the preferred view for determining truncal valve morphology and number of leaflets (Fig. 18.5). It is an excellent window to view the VSD in the outlet septum. The pulmonary valve, main pulmonary artery, and branches are absent from their usual location. Visualization of the pulmonary arteries arising from the common truncus is a key finding in differentiating this anomaly from pulmonary atresia with VSD. The coronary artery origins and proximal courses are usually best imaged from the parasternal short-axis views, with the right coronary artery originating from the anterolateral aspect of the truncal root and the left coronary artery originating from the left posterolateral aspect of the truncal root. In truncus arteriosus type I, the truncal vessel can be seen in cross section with a short main pulmonary artery segment originating from the lateral aspect and bifurcating into the right and left pulmonary arteries. In truncus arteriosus type II, the pulmonary artery branches can be seen emerging posteriorly from the truncal root, originating from close but separate orifices (Fig. 18.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


