Patients with functionally single ventricular circulations are challenges to evaluate by any method. The anatomy, physiology, and surgical procedures associated with these malformations are complex and varied. Conventional surgical palliation for the functionally single ventricle is usually focused toward completion of a Fontan “operation.” However, the Fontan circulation can be completed using several different surgical techniques. As a result, some of the confusion encountered when evaluating the post-Fontan patient stems from the number of different connections used to create the systemic venous to pulmonary arterial pathway. Therefore, a systematic approach is required if one is to truly understand the circulation of patients after Fontan operations. This chapter will discuss the anatomy of the Fontan circulation, its unique physiology, and an approach to the echocardiographic evaluation of these patients that not only allows a complete delineation of the Fontan circulation but also increases the likelihood of detecting late complications. In conclusion, a number of case studies illustrating the physiology and complications of this type of circulation will be reviewed.
ANATOMY AND PHYSIOLOGY OF THE FONTAN OPERATION
The first step toward understanding the patient with a Fontan circulation is to realize that this procedure is a surgical palliation, rather than a “repair.” A Fontan operation reduces ventricular workload and eliminates or reduces oxygen desaturation in the patient with a functionally univentricular heart. These goals can be achieved by many different surgical techniques, and the patients who can benefit from the Fontan operation have many different cardiac malformations. Thus, the Fontan is more of a surgical “concept” than it is a specific operative technique. This concept can be summarized as follows: A Fontan “operation” consists of any combination of surgical procedures that diverts the systemic venous return away from the ventricle, eliminates mixing of systemic and pulmonary venous blood, and creates a circulation “in series” for patients with functional single ventricle physiology. After creation of a Fontan, pulmonary venous flow remains committed to the patient’s only functional ventricle. Many types of pathways have been used to create the systemic venous diversion that defines the Fontan circulation. Regardless of how the connections were constructed, superior and inferior vena cava flows bypass the ventricle, and pass through the lungs without the assistance of a ventricular systolic pump.
When the Fontan procedure was first applied, the right atrium was used as the pathway for systemic venous flow, an anastomosis was created between the right atrial appendage, and the pulmonary artery provided the outlet from the atrium to the pulmonary arteries. This type of connection is referred to as an “atriopulmonary” Fontan and is illustrated in Figures 41.1 and 41.2. This approach worked well for patients with tricuspid atresia and others with left atrioventricular valves. However, this approach was difficult to apply to hearts with left atrioventricular valve atresia, hypoplasia, or severe left atrioventricular valve dysfunction. Although atriopulmonary connections have been superseded by newer techniques, there are many patients who still have these connections. Therefore, we need to not only understand how to recognize them, but also be aware of the unique complications associated with this connection. These complications include atrial enlargement and arrhythmias, compression of the pulmonary veins by the enlarged right atrial chamber, and atrial thrombus formation within the dilated atrium. Atrial thrombi, sluggish flow in a dilated atrial chamber (with spontaneous echo contrast), blind pouches in communication with the “left” heart (such as a ligated native pulmonary root), and residual right-to-left shunts have all been associated with an increased risk for embolic events.
Over time, less of the right atrium was included in the systemic venous pathway, allowing the right atrioventricular valve to contribute to the systemic circulation. Lateral atrial tunnels or intraatrial conduits allowed the pulmonary venous return to pass through any atrioventricular valve to reach the ventricle, expanding the spectrum of the Fontan to those with common atrioventricular valves and left atrioventricular valve abnormalities. Recently, the surgical systemic venous diversions involved in Fontan procedures have bypassed the atrium completely. This has been accomplished by combining direct superior vena cava–to–pulmonary arterial anastomoses (bidirectional Glenn connections) with intracardiac or extracardiac conduits. These nonvalved conduits connect the suprahepatic inferior vena cava to the pulmonary arteries (Fig. 41.3). A Fontan circulation that includes an extracardiac conduit has been referred to as an “extracardiac Fontan” (Figs. 41.4 and 41.5.
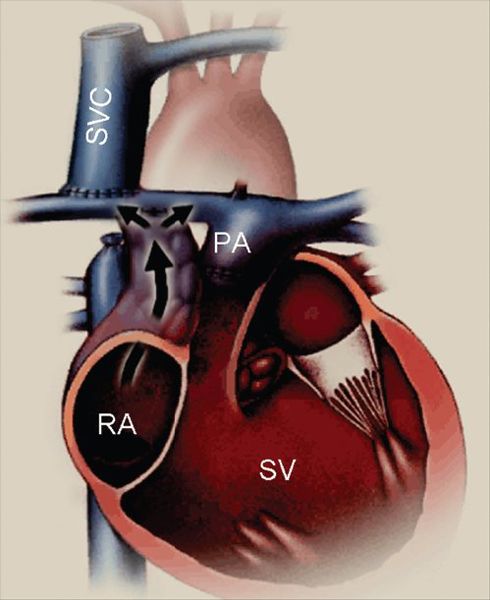
Figure 41.1. Diagram of an atriopulmonary Fontan connection. The underlying anatomy is that of a functionally single ventricle chamber, with right atrioventricular valve atresia and pulmonary stenosis. In this case, the superior vena cava (SVC) has been directly and bidirectionally connected to the right pulmonary artery (a “bidirectional Glenn” anastomosis). The native right atrium (RA) has been converted into a conduit for inferior vena caval flow by closure of the atrial septal defect and a right atrial appendage-to–pulmonary artery (PA) connection (black arrow). Early atriopulmonary Fontan connections often did not involve a separate SVC connection as shown here. Both of the venae cavae were left connected to the RA, and the atriopulmonary anastomosis carried all of the systemic venous return. The use of the native RA for at least part of the venous pathway is the hallmark of an atriopulmonary Fontan. The elevated venous and right atrial pressures associated with the Fontan circulation lead to prominent right atrial enlargement after this type of connection. SV, functional single ventricle.
Since the early 1990s, many Fontan operations have included a surgical “fenestration.” A fenestration is essentially a small, intentional residual atrial septal defect (Figs. 41.3 and 41.6). Atrial baffle fenestrations are usually sized between 3 and 5 mm and allow a small, continuous right-to-left atrial shunt, at the expense of mild systemic oxygen desaturation. The physiology of the fenestration’s shunt is always right to left. This is because of the absence of a ventricle in the right side of the circulation. As a result, the right atrial/pulmonary arterial pressure must be greater than the functional left atrial/pulmonary venous pressure, or there would be no driving force for pulmonary blood flow. The purpose of allowing this residual shunt via an atrial fenestration is to provide a relatively continuous source of preload to the systemic ventricle. Prior to creation of a Fontan circulation, functionally single ventricles universally have an increased preload, since they are “filled” by both the systemic and pulmonary venous returns. At the time of Fontan creation, this extra filling volume is suddenly reduced, by diverting all of the systemic veins to the pulmonary arteries. The extra volume provided to the ventricle by a fenestration eases the transition to the Fontan circulation and maintains a slightly higher cardiac output reserve even after the initial postoperative adjustment period. Fenestration has been shown to reduce the duration of pleural drainage in the immediate postoperative period. The disadvantage associated with a fenestration is that patients will remain cyanotic and therefore have a slightly increased risk for embolic complications compared with the nonfenestrated patient.
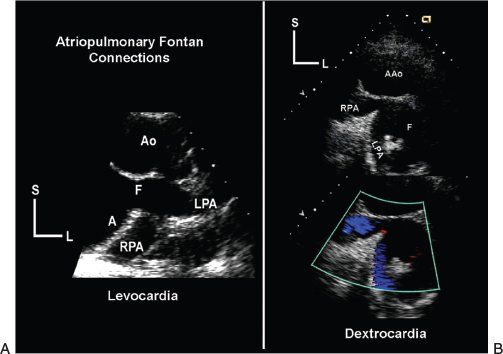
Figure 41.2. Parasternal, horizontal-plane echocardiographic images demonstrate the tomographic anatomy of atriopulmonary Fontan connection. A: Taken during an examination of the patient with normally positioned atria and levocardia. The atrial appendage has been surgically opened and connected to the pulmonary arterial confluence, creating the Fontan connection (F). These connections are found posterior to ascending aorta, just superior to the base of the heart. B: Taken during the examination of the patient with atrial situs inversus and dextrocardia. As a result, the anatomy is a mirror image of the left panel. These images were obtained from the right parasternal border. Scans in the horizontal plane began by demonstrating the dilated native right atrial chamber. The plane of sound was then progressively moved superiorly beyond the semilunar valve to reveal the Fontan connection (F) and the confluence of the right and left pulmonary arteries (RPA and LPA). Regardless of how the surgeon created the connection, a similar progression of scans (beginning with the atrium and moving toward the pulmonary arteries) should allow echocardiographic demonstration of the Fontan anastomosis. A, atrium; AAo or Ao, ascending aorta; L, left; S, superior.
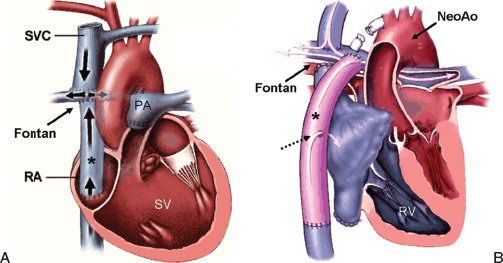
Figure 41.3. Diagrams of two types of Fontan circulations that completely bypass the entire heart to create the diversion of superior and inferior vena caval flow to the pulmonary arteries. A: This panel illustrates the use of a nonvalved conduit to complete the Fontan pathway. In this case, the base of an intraatrial conduit was connected to the inferior vena caval–atrial junction. The superior end of the conduit was then connected to the pulmonary arterial confluence using an incision/anastomosis at the dome of the native right atrium. Although the atrial wall is used to complete the connection, none of the native atrial chamber is actually involved in the venous pathway. The superior vena cava (SVC) has a separate, bidirectional anastomosis to the right pulmonary artery. B: Anatomy of an extracardiac, fenestrated Fontan operation performed in a patient with prior Norwood reconstruction for hypoplastic left heart syndrome. In this case, the inferior vena cava was detached from its native connection to the right atrium and a direct anastomosis was made between the supra-diaphragmatic inferior vena cava and a nonvalved conduit (asterisk). A superior connection was then made between the conduit and the pulmonary arterial confluence. A 4-mm fenestration (dashed, black arrow) was created between the conduit and the lateral wall of the native atrium. This allowed a small residual right-to-left shunt (white arrow) to provide a continuous source of extra ventricular filling (preload), easing the transition to the Fontan circulation. Two operations had been performed prior to the Fontan completion, as described earlier. The aorta had been reconstructed during the neonatal Norwood operation, enlarging the aortic arch and fusing the hypoplastic native ascending aorta with the native pulmonary root to create a “neoaorta.” The SVC had been bidirectionally connected to the right pulmonary artery at an intermediate, second-stage operation. PA, pulmonary artery; RA, native right atrium; RV, right ventricle; SV, functionally single ventricle.
From an echocardiographer’s perspective, a fenestration also provides insight into the patient’s pulmonary hemodynamics. The mean pressure gradient across the fenestration can be easily measured by continuous-wave Doppler echocardiography (Fig. 41.6). The fenestration “shunt” originates in the functional right atrium (the Fontan pathway), and (in the absence of stenoses) the pressure in the Fontan pathway is equal to the pulmonary arterial pressure. The fenestration flow is directed into the functionally left atrium. In the absence of pulmonary vein stenosis, the pressure in the functional left atrium will equal the pulmonary venous pressure. Therefore, the mean pressure gradient across the fenestration will reflect the “transpulmonary gradient.” This gradient is primarily determined by the patient’s pulmonary vascular resistance, a key determinant of outcome in patients with Fontan circulations. Fontan circulations that are functioning well are associated with mean fenestration (transpulmonary) gradients of 5 to 8 mm Hg. Lower values may represent better than average Fontan physiology or may represent dehydration with artificially low right atrial pressures. The higher the gradient, the higher is the total transpulmonary resistance to flow. Gradients greater than 8 mm Hg or that have increased from the patient’s historical baseline require explanation, prompting an even more thorough evaluation than usual.
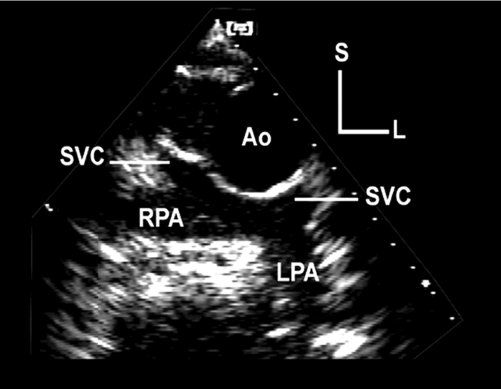
Figure 41.4. Horizontal-plane echocardiographic image from a high left parasternal, subclavicular position. The image demonstrates bilateral, bidirectional superior vena cava (SVC)–to–pulmonary artery connections. These anastomoses are often referred to as bidirectional Glenn connections or shunts. This type of connection can be used as a part of a Fontan circulation, diverting superior vena caval blood flow away from the heart and into the pulmonary arteries. These connections are often created prior to final Fontan completion, in an attempt to minimize ventricular volume overload in very young patients who would otherwise not tolerate creation of a complete Fontan. In more mature patients, if these connections are not already present, they can be made at the time of Fontan completion. Ao, ascending aorta; L, left; LPA, left pulmonary artery; RPA, right pulmonary artery; S, superior.
The physiology of the Fontan operation is unique, primarily because the redirected venous flow streams do not benefit from a ventricular pump. Forward flow through the lungs depends upon a combination of residual kinetic energy from the “previous” systemic ventricular contraction, negative intrathoracic pressure (generated by the respiratory muscles), low pulmonary arterial pressure and resistance (involving both large and small vessels), as well as active atrial and ventricular relaxation. Several of these influences are reflected in the pulmonary arterial flow patterns recorded by Doppler echocardiography in Fontan patients (Figs. 41.7 and 41.8). The respiratory influence on flow is reflected by the marked augmentation in the Doppler signal occurring during inspiration. The negative intrathoracic pressure generated during spontaneous inspiration also draws blood forward through the lungs. Conversely, positive intrathoracic pressures, seen in expiration or with mechanical ventilation, will reduce forward flow. Active ventricular diastolic relaxation also serves to augment forward flow through the lungs. As the atrioventricular valve(s) open, forward flow increases through the pulmonary arteries (Figs. 41.7 and 41.8). Reduced ventricular compliance and elevated ventricular diastolic pressure will blunt this flow, reducing overall cardiac output, especially the ability to increase output with activity (cardiac reserve). Left atrial mechanical activity also affects pulmonary arterial flow in Fontan circulations. Atrial relaxation will augment forward flow by drawing blood out of the pulmonary veins, increasing overall forward flow. In contrast, atrial contraction will generally somewhat decrease the forward flow signal. If the ventricle has good diastolic compliance, the increase in pulmonary venous pressure caused by atrial contraction is offset by the increase in ventricular filling and output associated with synchronous atrial rhythms, such as normal sinus rhythm and dual chamber pacing. However, reduced ventricular compliance with elevated diastolic pressure or the atrial contraction is not synchronized with ventricular relaxation (as in junctional rhythms or heart block) will inhibit forward flow and impair cardiac output. Pulmonary venous pressures and flow reversals associated with atrial contraction in nonsynchronous rhythms are dramatically increased, reducing forward flow (Fig. 41.9).
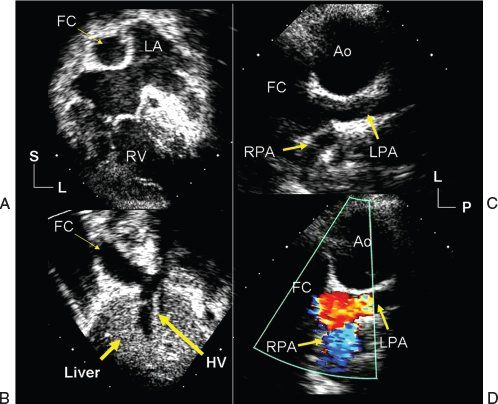
Figure 41.5. Echocardiographic images demonstrate the appearance of an extracardiac Fontan operation in a patient with hypoplastic left heart syndrome. A: Taken at the cardiac apex; demonstrates how the native left atrial (LA) and right atrial (not labeled) chambers now serve as a combined pulmonary venous chamber, while the extracardiac, Fontan conduit (FC) functions as the patient’s new right atrium. B: Inferior connection of the Fontan conduit to the inferior vena cava and hepatic venous confluence (HV). C: Superior connection of the Fontan conduit to the pulmonary arterial confluence in a coronal plane. The scans were obtained from the left anterior axillary line with the plane of sound directed toward the patient’s right. The Fontan connection and pulmonary arteries are seen just beyond the reconstructed ascending aorta (Ao). There was no evidence of narrowing within the venous pathway or pulmonary arteries. Color flow Doppler interrogation (D) revealed laminar flow consistent with widely patent connection from the inferior vena cava, through the Fontan conduit to the pulmonary arteries. L, left; LPA, left pulmonary artery; P, posterior; RPA, right pulmonary artery; S, superior.
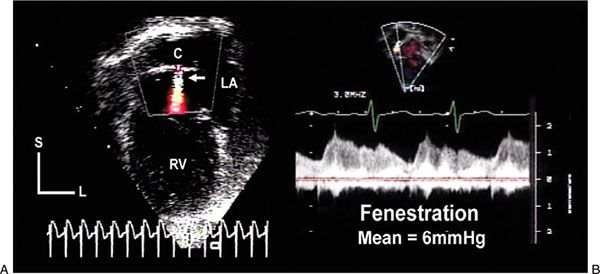
Figure 41.6. Echocardiographic images demonstrate an atrial fenestration after completion of a Fontan operation in a patient with asplenia syndrome. A: An intraatrial conduit (C) diverted inferior vena caval flow to the pulmonary artery. Color flow Doppler showed a continuous, aliased jet of flow (white arrow) from the conduit into the pulmonary venous atrial chamber (LA). B: Continuous-wave Doppler interrogation revealed this flow pattern. Velocities varied during each phase of the cardiac cycle and with respiration. The mean gradient between the conduit and the pulmonary venous atrium was 6 mm Hg, representing a relatively normal transpulmonary gradient after Fontan completion. L, left; RV, right ventricle; S, superior.
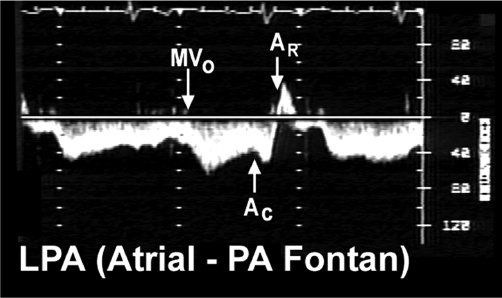
Figure 41.7. Pulsed-wave Doppler recording was obtained in the left pulmonary artery of the patient with tricuspid valve atresia and an atriopulmonary Fontan connection. The tracing demonstrates three important phases to “Fontan” flow in this type of connection. Forward flow (below the baseline) is augmented by active ventricular diastolic relaxation when the systemic atrioventricular valve opens (MVO). Since the native right atrial chamber remains in the systemic venous pathway, atrial contraction will also increase forward flow velocity (AC). However, when the atrium relaxes (AR), flow actually reverses out of the pulmonary artery and returns to the atrium (signal now shown above the baseline). This to-and-fro flow contributes to the atrial enlargement seen with this type of Fontan connection. Longer recordings would also demonstrate a respiratory influence on these flows, such as negative intrathoracic pressure (caused by spontaneous inspiration) that will increase forward flow volume and velocity in the Fontan circulation. In contrast, positive expiratory pressures will blunt forward flow. AC, atrial contraction; AR, atrial relaxation; MVO, mitral valve opening; LPA, left pulmonary artery; PA, pulmonary artery.
In the setting of an atriopulmonary Fontan connection, the native right atrial contraction and relaxation also alter the pulmonary arterial flow pattern. However, unlike with the left atrium, right atrial mechanical activity is inefficient in a Fontan circulation and does not actually alter cardiac output. Any augmentation to forward flow caused by right atrial contraction is counteracted by the accompanying reversal (from the pulmonary artery back into the right atrium) that occurs during atrial relaxation (Fig. 1.7). The only real impact of right atrial contraction in these patients is to create a “to-and-fro” flow through the Fontan connection, which contributes to the progressive atrial dilation common to this type of connection.
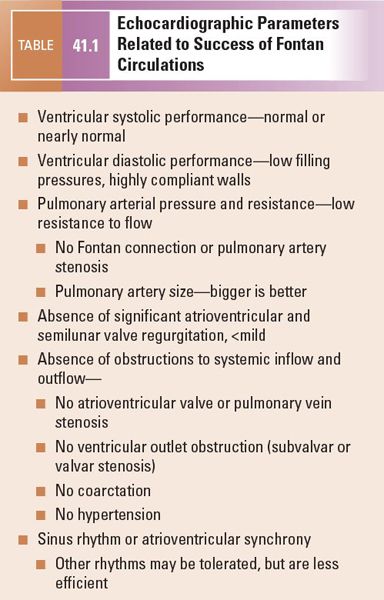
The determinants of pulmonary blood flow just described provide clues to factors that identify successful Fontan patients and some of the complications that are poorly tolerated by those with Fontan circulations. Ventricular function (both systolic and diastolic) and pulmonary resistance are probably the most critical variables related to the success and/or failure of any Fontan circulation. Table 41.1 outlines these and other factors that combine to create favorable Fontan circulations. If there are multiple negative factors, the patient is likely to struggle after the Fontan and is much more likely to develop significant complications.
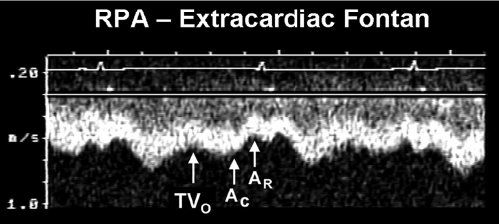
Figure 41.8. Pulsed-wave Doppler recording obtained from the right pulmonary artery of the patient with hypoplastic left heart syndrome and an extracardiac Fontan connection. This tracing also demonstrates phasic flows, but there are important differences to note relative to the flow patterns seen in Figure 41.7. Ventricular diastole and atrioventricular valve opening result in augmented forward flow in both types of Fontan connections. In this recording, this is reflected by the increased forward flow seen in early diastole (TVO). There is no atrial tissue in the extracardiac Fontan pathway, therefore the to-and-fro signal seen in Figure 41.7 is not evident. However, left atrial activity can still influence the pulmonary arterial flow pattern. When the “left” (pulmonary venous) atrium contracts (AC), pulmonary venous pressures rise slightly. This reduces forward flow velocity in the pulmonary artery somewhat. In an extracardiac Fontan, left atrial relaxation (AR) promotes forward flow by drawing blood out of the pulmonary veins and into the atrium. Although pulmonary arterial flow is still phasic in an extracardiac Fontan, the normal flow velocity should rarely decrease to near zero. This is unlike the flow seen in an atriopulmonary Fontan connection, where even flow reversals are common (Fig. 41.7). Intrathoracic pressure changes will produce the same alterations in these flow patterns that were described for the atriopulmonary Fontan connection in Figure 41.7. RPA, right pulmonary artery; TVO, tricuspid valve opening.
IMAGING STRATEGIES FOR EVALUATION OF PATIENTS AFTER FONTAN OPERATIONS
Most Fontan patients begin their postoperative follow-up at an older age than other patients with complex congenital heart disease. This is because of the staged nature of this surgical palliation. The fact that Fontan completion was historically performed at even older ages also contributes to this shift in demographics. As a result, the difficulties in imaging these patients are twofold. The examiner faces not only the complexity of the surgical procedure and underlying congenital heart disease, but also the reduction in image quality that is associated with increasing age and multiple prior surgical procedures. Nevertheless, the mainstay of cardiac diagnostic imaging continues to be transthoracic echocardiography. This technique provides convenience, reproducibility, and wide availability. Transesophageal echocardiography, magnetic resonance imaging, computerized tomography, and angiography all play key, but secondary, roles in obtaining structural and functional information in this patient group. These supplemental imaging strategies should be used when the clinical information required is not adequately outlined by the surface echocardiogram. The most important time to add these alternative imaging techniques to a patient’s evaluation is when he or she is clinically deteriorating, even if the changes are small.
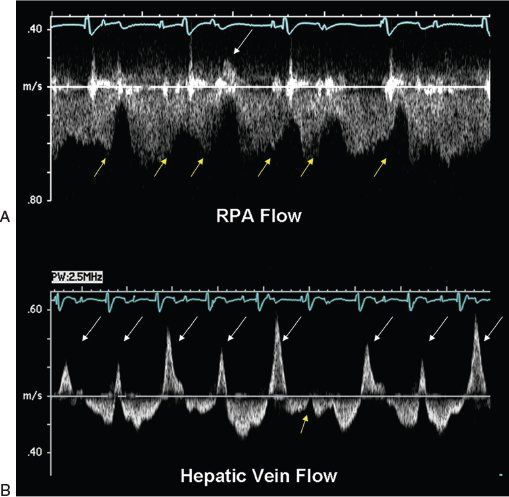
Figure 41.9. Pulsed-wave Doppler recordings demonstrate how cardiac rhythm disturbances can also influence flows within the Fontan circulation. These signals were obtained from the right pulmonary artery (A) and the hepatic vein (B) of a patient after an extracardiac Fontan operation. These flow patterns are not normal, primarily because of the abnormal cardiac rhythm that is present. Electrocardiography reveals complete heart block with a junctional escape rhythm. The atrium is contracting more often than the ventricle in this case. Since atrial contractions are dyssynchronous in this rhythm, they will produce significant elevations of pulmonary venous pressure (cannon A waves). These pressure increases are reflected not only in the pulmonary arterial flow signal, but are also transmitted all the way back to the hepatic veins (even though there is no “atrium” within the systemic venous pathway). A, yellow arrows: Reductions in forward flow velocity caused by atrial contraction. One of the atrial contractions during this recording occurred so early (white arrow) that it actually caused flow reversal in the pulmonary artery. This phenomenon was much more evident when the hepatic venous flows were recorded (B). Cannon A waves with large flow reversals (white arrows) could be seen during nearly every cardiac cycle (yellow arrow). The one cardiac cycle in this recording in which atrial contraction occurred at approximately the “correct” time was relative to ventricular contraction. In this cardiac cycle, there was a slight decrease in forward velocity after atrial contraction, but no reversals were observed.
Knowledge of the patient’s clinical status is also helpful to choosing an imaging strategy. For example, the patient with increasing fatigue must be evaluated for worsening ventricular systolic or diastolic performance, as well as arrhythmias. The patient with a recent stroke or transient ischemic accident must have a detailed search for the source of the emboli. While imaging these patients, one must strive to obtain detailed, high-quality images. Unfortunately, we know that after the Fontan procedure, patients often have challenging acoustic windows. When surface echocardiography does not provide adequate detail, transesophageal echocardiography is often useful in visualizing the anatomic areas in question. Transesophageal echocardiography is particularly well suited to evaluating posterior structures such as the atria (to exclude thrombus formation), the Fontan connections (to exclude obstruction), and the atrioventricular valves. Magnetic resonance imaging can be helpful in identifying venous or arterial abnormalities and in quantitating ventricular function, assuming the patient does not have an electronic pacemaker. Cardiac catheterization and angiography still play an important role in the evaluation of a patient’s hemodynamic status after the Fontan operation. Catheter-derived hemodynamics remain the gold standard for determination of precise pressure measurements for comparison to the patient’s historical baseline and pulmonary vascular resistance.
We will focus the remainder of this chapter on surface echocardiographic evaluations, supplementing the discussion with examples of more invasive imaging strategies, when appropriate.
IMAGE ACQUISITION AND THE ANATOMY OF THE FONTAN RECONSTRUCTION
The most important tool in any assessment of a Fontan patient is the surgical dictation. To perform an adequate examination, one must know exactly how the Fontan circulation was created. Early atriopulmonary connections often used the right atrial appendage as the final portion of the pathway for systemic venous flow (Figs. 41.1 and 41.2). The atrial septum was closed in its natural position, and any atrioventricular connection from the right atrium to the ventricle was also closed. Similarly, the pulmonary artery, if present, was ligated and/or divided. Although the atriopulmonary Fontan achieves separation of the systemic and pulmonary venous flow streams, it leaves a large and distensible chamber (the right atrium) in the middle of the systemic venous pathway. Progressive right atrial enlargement, intraatrial thrombi, and persistent atrial arrhythmias have plagued the post-Fontan patient with this type of connection. Intracardiac thrombi can be detected by surface echocardiography (Fig. 41.10). However, transesophageal examinations are more effective in detecting these complications (Fig. 41.11), especially since the transthoracic image quality available in an older Fontan patient is often impaired.
As a result of these problems, more recent surgical connections have been modified and become more streamlined. The most common method used to create a Fontan circulation today combines bidirectional superior vena cava to pulmonary arterial anastomosis(es) with an extracardiac conduit, creating continuity between the inferior vena cava and the pulmonary artery. This type of connection, the extracardiac Fontan, is illustrated in Figures 41.3, 41.4, and 41.5. Not only does an extracardiac Fontan eliminate the dilated right atrial chamber, but it also allows use of both native atria and both atrioventricular valves in the systemic circulation. This approach simplifies creation of a Fontan circulation for patients with anomalous pulmonary venous connections and abnormalities of the atrioventricular valve(s).
These two types of Fontan pathways do not represent the only Fontan connections that are possible. This heterogeneity in surgical approach contributes to making the surgical dictation so valuable. The surgical report also allows the examiner to be confident at the conclusion of the study that all of the components of the Fontan have been assessed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


