Fig. 1.1
A cross-sectional view of the atrioventricular canal of an E13 mouse heart showing the superior (sAVC), inferior (iAVC), and right (rlAVC) and left (llAVC) lateral atrioventricular cushions. The principal cushions are beginning to fuse together centrally (Reproduced from Snarr et al. [2] with permission)
In the sixth week, the atrioventricular canal begins to expand rightward at the atrial end, becoming funnel-shaped, so that it underlies the developing right atrium as well as the left atrium [4]. The ventricular end continues to communicate only with the developing left ventricle. Blood enters the developing right ventricle only through the outlet or interventricular foramen. During the seventh week, rightward expansion of the canal and enlargement of the cushions continue. The atrial septation complex, consisting of septum primum, or the primary atrial septum, with its mesenchymal cap, and the dorsal mesenchymal protrusion, approach the principal atrioventricular cushions, closing the ostium primum or primary interatrial foramen [5]. By now the rightward portion of the atrioventricular canal is aligned with the right ventricle as well as the right atrium because of rapid expansion at the ventricular end across the interventricular foramen [4]. As the atrial septation complex makes contact with the two principal cushions, they fuse together, as well as with the atrial septum, dividing the atrioventricular canal into right and left portions which connect the ipsilateral atrium and ventricle [6] (Fig. 1.2). The right atrioventricular orifice is substantially smaller than the left but will expand with growth of the right lateral cushion. In the seventh week, the fused principal cushions become draped over the muscular inflow septum which has formed between the bases of the ventricles [6] (Fig. 1.2). The right lateral cushion, which will form the superior (anterior) and inferior (posterior) leaflets of the tricuspid valve, has enlarged to cover much of the lateral aspect of the expanding right atrioventricular junction [2]. The right side of the fused principal cushions, which will form the medial or septal leaflet, has now become adherent to the right side of the ventricular septum. The right lateral cushion elongates into the right ventricular cavity on a skirt of the atrioventricular canal myocardium interdigitating with the ventricular myocardium [6]. The atrioventricular canal myocardium beneath the cushion gradually undergoes apoptosis, freeing up the thinning and elongating superior and inferior leaflets. Muscular connections with the ventricular myocardium remain at the free edge as papillary muscle attachments. The medial leaflet begins to delaminate from the ventricular septum after the mural leaflets have begun to form, also by apoptosis of the underlying myocardium. This process continues even after the completion of embryogenesis, at least into the ninth and tenth weeks. Valve leaflets continue to thin, elongate, and increase in circumference concomitant with growth of the ventricle. However, the laminar structure of leaflets does not develop until after birth.
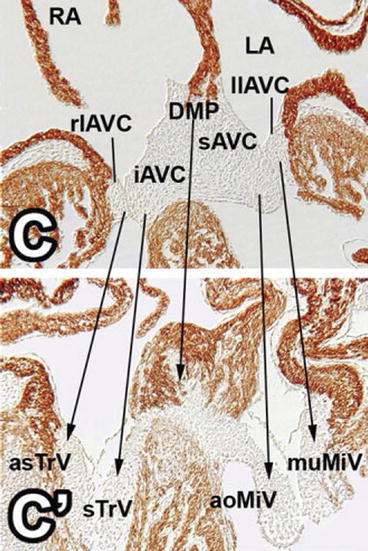

Fig. 1.2
A four-chamber view of embryonic mouse hearts: upper panel (C) – at a similar stage to Fig. 1.1 showing the fused superior (sAVC) and inferior (iAVC) atrioventricular cushions which are also fused with the dorsal mesenchymal protrusion (DMP) of the atrial septation complex. The fused principal cushions are becoming draped over the inflow portion of the ventricular septum. The left (llAVC) and right (rlAVC) lateral cushions are present but have not begun to enlarge. Lower panel (C’) – at E14.5 showing development of the structures seen in the upper panel. All of the cushions have elongated into the developing ventricular chambers. The septal tricuspid leaflet (sTrV) is now adherent to the right side of the ventricular septum, while the medial mitral leaflet (aoMiV) is unsupported and free in the left ventricle. The lateral cushions have lengthened into the ventricles on a sheet of atrioventricular canal myocardium. The dorsal mesenchymal protrusion has muscularized to become the base of the atrial septum, below which the fused central part of the principal cushions is forming the membranous septum and central fibrous body. RA right atrium, LA left atrium, asTrV anterior superior tricuspid valve leaflet, muMiV mural mitral valve leaflet (Reproduced from Snarr et al. [2] with permission)
The valve annulus forms around the free wall by ingrowth of fibroadipose sulcus tissue at the atrioventricular groove between the ventricular and atrioventricular canal myocardium, finally reaching the base of the forming valve leaflets [7] (Fig. 1.3). The septal portion of the valve ring is formed by the fused central portion of the principal cushions and is continuous through the membranous ventricular septum and central fibrous body with the septal insertion of the mitral valve [6, 8]. The annulus is an important component of the fibrous skeleton of the heart and electrically isolates the atrial from the ventricular myocardium. The valve leaflets and chordae tendineae derive predominantly, if not completely, from the mesenchymal cells of the cushions, themselves derived from endothelium [6, 8] (Fig. 1.4). Papillary muscles derive from the ventricular myocardium that initially underlay the edges of the developing cushions.
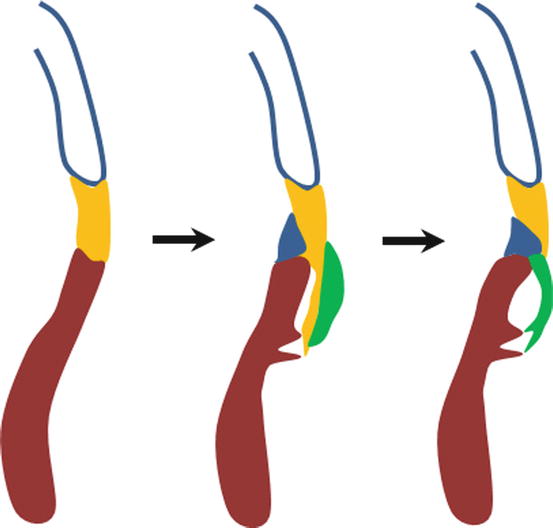
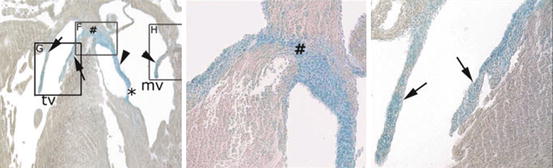

Fig. 1.3
Cartoon illustrating development of the fibrous annulus of the tricuspid valve which separates and insulates the atrial and ventricular myocardium. In the left panel, the atrial (blue outline) and ventricular (brown) myocardia are connected by the atrioventricular canal myocardium (yellow). In the middle panel, the sulcus tissue (blue) has invaginated into the atrioventricular groove and the lateral cushion (green) has lengthened into the ventricle on a sleeve of the atrioventricular canal myocardium. In the right panel, the sulcus tissue has separated the atrioventricular canal myocardium from the ventricular myocardium by making contact with the mesenchyme of the developing valve leaflet. The atrioventricular canal myocardium under the developing leaflet has undergone apoptosis leaving the thinning and lengthening leaflet free. The remaining atrioventricular canal myocardium has been sequestered in the atrium forming the smooth vestibule of the tricuspid valve

Fig. 1.4
Neonatal mouse heart showing: left, thinned and elongated atrioventricular valve leaflets stained blue for an endothelial lineage marker (Tie2 Cre); middle (box F), the fibrous connection between the medial leaflets of the tricuspid and mitral valves (#), also derived from endothelial cells; right (box G), tricuspid valve leaflets (arrows) with the medial tricuspid leaflet delaminated from the septum. Arrows heads mitral valve leaflets, # central fibrous body connecting the medial tricuspid and mitral leaflets, mv mitral valve, tv tricuspid valve (Reproduced from Lincoln et al. [8] with permission)
Extracellular matrix protein expression is locally restricted during remodeling of the valve primordia into formed leaflets [8]. Type I collagen is the predominant type expressed in the developing fibrous leaflets. In contrast, type II collagen, usually associated with cartilage, is present in the chordae tendineae, but not in leaflet. Tenascin, characteristic of tendon and cartilage, is expressed in both valve leaflet and chordae tendineae. Scleraxis, a transcription factor in the tendon and cartilage gene regulatory network, is expressed only in chordae, particularly near the myotendinous junction. Not only is valve maturation molecularly compartmentalized, it also bears remarkable similarity to skeletal development.
The adult trilaminar arrangement of the valve leaflets develops postnatally [9]. Collagen fibers become densely packed on the ventricular side of the leaflet forming the fibrosa which strengthens the leaflet and resists stretching. The atrial surface of the leaflets, the atrialis, contains numerous elastic fibers. The central layer or spongiosa is rich in glycosaminoglycans and versican and is thought to absorb energy associated with closure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


