Fig. 5.1
Downward displacement of the functional annulus (FA) from the anatomical annulus (AA). The displacement produces the atrialization of a portion of the right ventricle (aRV). CS coronary sinus
The disease may have a clinical neonatal presentation which corresponds to a severe RV and TV dysfunction or may present later in life, concurrently with an increasing degree of TV regurgitation and RV dilatation.
Since the pioneer period of cardiac surgery, many techniques have been described to address the TV and RV anomalies, including TV replacement which is still an option, despite reconstructive techniques being now preferred due to the improvement of the early and long-term results.
The aim of the repairing techniques is to restore a normal tricuspid valve function, to preserve the right ventricular contractility and to decrease the risk of rhythm disturbances.
Results are associated with the severity of the disease, the surgeon experience, and the perioperative care of the patient.
5.2 Anatomy
EA is defined by a spectrum of anomalies:
(a)
Apical displacement of the septal and part of the posterior leaflets for at least 8 mm/m2 or for 15 mm in infants and 20 mm in adults [6]
(b)
Atrialization of a portion of the right ventricle
(c)
Adhesions of TV leaflets to the right ventricular endocardium
(d)
Anomalies of the anterior leaflet
(e)
Dilation of the anatomical annulus
The final result of these anomalies is TV regurgitation. The degree of TV regurgitation is correlated with the importance of the displacement of the posterior and septal leaflets and also to the motion of the anterior leaflet, which can be impaired.
The septal leaflet is displaced in the RV, and the commissure between the septal and the posterior (inferior) leaflet is situated downward inside the RV. The posterior leaflet could be absent, displaced, or normal. When the posterior leaflet is absent or totally adherent, a bridge of tissue valve is present between the anterior leaflet and the septal one, in the apex of the RV. The anterior leaflet is mostly normally implanted but the motion is impaired, and the leaflet could present fenestrations or could be attached linearly to the endocardium. Muscular adhesions are present between the ventricular face of the leaflet and the infundibulum, reducing the motion. The TV insufficiency is correlated with these adhesions which tethered the leaflet.
The rotation of the annulus toward the right ventricular outflow tract creates an atrialization of a portion of the right ventricle (aRV). The aRV could present with a thin and fibrotic wall which is acting as an aneurysmatic sac. The annulus of the TV is always enlarged (Fig. 5.2).
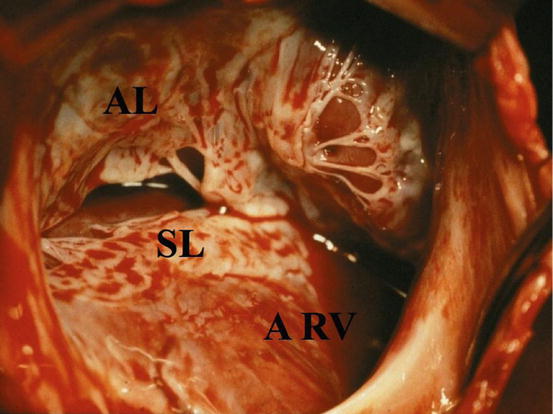

Fig. 5.2
Operative view from the first surgeon eyes: patient with the type C Ebstein anomaly. SL septal leaflet, AL anterior leaflet, ARV atrialized right ventricle
The right ventricle is myopathic and has a thinner wall and reduced number of muscle fibers; thus, dilation occurs not only on hemodynamic basis [7]. Also the left ventricle is involved presenting systolic and diastolic dysfunction. This could be related to the anomalous geometry of the right ventricle and to the oxygen desaturation causing interstitial fibrosis [8]. LV function is sometimes difficult to calculate as the LV could be underfilled or compressed by the RV.
In 1988, Carpentier and colleagues [9] proposed a classification based on the severity of the anatomical features and described four types (Fig. 5.3):
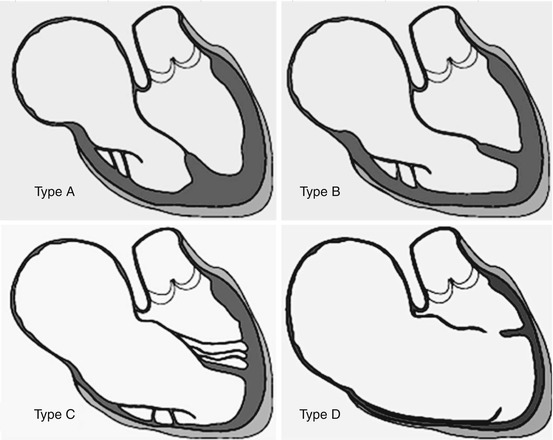

Fig. 5.3
Carpentier’s classification: type A minor anomaly, the atrialized RV is small; type B intermediate, the displacement of the septal leaflet is 2–3 cm, and the atrialized RV is normally contracting; type C severe form, the septal leaflet is severely displaced, the posterior leaflet is adherent to the ventricular wall or absent, and the atrialized right ventricle is huge with hypo- or akinetic motion; and type D tricuspid sac, the leaflet tissue, even the anterior one, is adherent to the RV wall. The contractility of the RV is globally impaired
Type A: Anterior leaflet is large and has a free leading edge. Septal leaflet is poorly displaced, the ventricular cavity is almost normal, and there is a small atrialized portion of the RV.
Type B: The displacement of the septal leaflet is obvious on echo and as deep as 2–3 cm; the anterior leaflet moves normally. The atrialized chamber is large and poorly contractile.
Type C: Massive displacement of the septal leaflet with muscular adhesions of the anterior leaflet to RV wall. Atrialized RV is huge and not contractile.
Type D: All the leaflet tissue is adherent to the ventricular wall which is thin and poorly contractile. The RV is made by atrialized chamber.
According to Carpentier’s classification, the patients are distributed mainly in types B and C, accounting for 50 and 30 % of the patients, respectively, and finally in types D and A, accounting for 10 and 8 %, respectively, [10].
Many associated lesions are usually described. The most common is an atrial septal defect (ASD) which is present in nearly 70 % of patients, then arrhythmic disorders in approximately 10 % of patients, and finally pulmonary atresia and VSD in 7 % of patients [10].
5.3 Symptoms
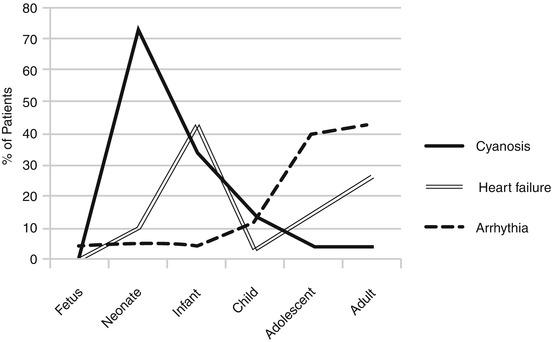
The three predominant symptoms are dyspnea, cyanosis, and arrhythmia. Their association is frequent. Dyspnea is most frequently due to the TV insufficiency which decreases the total cardiac output. Even when symptoms are absent, an exercise testing usually reveals a reduced functional capacity.
Cyanosis is related to right-to-left shunt across the interatrial communication and is present in 40–50 % of patients. The degree depends upon the severity of the disease and could be severe and sustained or increased by the TV regurgitation.
Arrhythmia is a disorder noticed by patients with short bouts of tachyarrhythmia and sometimes syncope. It is not frequently a preexcitation syndrome or flutter even if Wolff-Parkinson-White syndrome is present in 8–10 % of patients. Rhythm anomalies appear later if compared to the onset of dyspnea.
The valve regurgitation itself is responsible for TV annulus dilation, which is the cause of the increase of regurgitation in a vicious loop. This is the cause of heart failure. However, classical signs of right heart failure such as liver enlargement and lower limb edema are exceptional even with massive TV insufficiency.
When the RV is dilated, or poorly functional (hypokinesia), patients are symptomatic earlier in life. Nevertheless, the most common functional pattern of Ebstein in adolescents is the absence of massive dilatation of the RV. Thus, the adolescents and young adults have generally good ventricular function and mild symptoms which could not be correlated to the degree of TV regurgitation and which are mostly related to arrhythmic disorders.
Presence of the ASD is a predictor of possible paradoxical embolism which might occur during the life of the patients with EA. The embolism is the cause of neurological damage, even stroke or transient ischemic attack or brain abscess and myocardial infarction. It is reported to occur in more than 20 % of adult patients [51].
Death usually occurs early in life with a rate of 13 % in the first month and 17 % in the first year after EA diagnosis. Subsequently, the risk decreases and probability to survive is 80 % after 3 years and remains stable during childhood. Older children have a significantly better outcome than newborns. Young age, hepatomegaly, the need for mechanical ventilation, medical treatment, and other associated cardiac anomalies at the time of EA diagnosis are predictors of death [12] (Fig. 5.4).
5.4 Diagnosis and Preoperative Evaluations
At the present time, in developed countries, the diagnosis is performed very early after birth or during fetal life.
Diagnosis with echocardiography is usually easy (Fig. 5.5), detecting the valve deficiency and its morphological typical patterns. Right cavities are enlarged proportionally to the degree of TV insufficiency. The RV contractility must be assessed, and tools for that are not very precise. 2D echocardiography has some well-known limitation for calculating RV volume and ejection fraction [13].
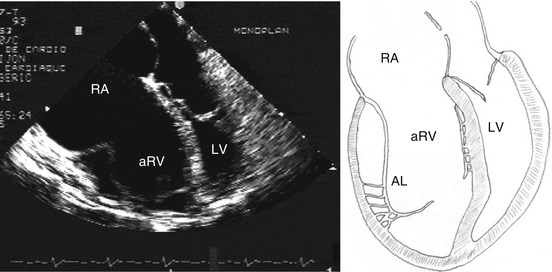

Fig. 5.5
Echocardiogram: four-chamber view and schematic drawing of the echocardiographic view. The left atrium and the left ventricle are compressed by the right chambers. RA right atrium, aRV atrialized right ventricle, LV left ventricle, AL anterior leaflet
In the past years with the purpose of overcoming the limit of the echocardiography, the morphological evaluation of the valve, of the atrialized portion of the RV, and of the RV has been accomplished using other imaging techniques such as computed tomography (CT) scan or magnetic resonance (MRI) (Fig. 5.6). CT scan has been adopted and proved to be efficient to evaluate changes in ventricular volume in conservative surgery for EA [14] but subjects the patient to radiation. MRI provides good morphological details and information obtained by echocardiography, and MRI is often comparable with the exception for the study of the posterior leaflet and RV function which are better evaluated by MRI [15–17]. Nevertheless, even cardiac MRI has failure to predict the RV myocardial reserve which is a clue prognostic factor. Recently the 3D echocardiography seems to reveal useful morphological details of TV and RV outflow tract before, during, and after surgery [18].
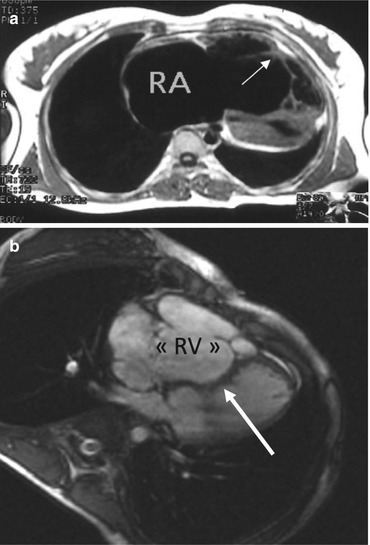

Fig. 5.6
Cine MRI. (a) Horizontal view showing the muscular attachment of the anterior leaflet (white arrow). (b) Paradoxical septum bulging in the left ventricle during systole (arrow). RV atrialized right ventricle. RA right atrium
Electrocardiography is essential since it is abnormal in most patients [19]. Usually a tall and broad P wave is present, and right bundle branch block is often observed. Furthermore, first-degree AV block is present in 40 % of patients.
Wolff-Parkinson-White syndrome is frequent and affects approximately 10 % of the patients [20]. Patients with preexcitation would have an advantage in electrophysiological mapping in order to localize the accessory conduction pathways either for a transcatheter or for a surgical ablation. However, the transcatheter ablation is often difficult because of atrial dilatation which alters the anatomical landmarks. Indeed the ablation in EA is less effective than in patients with normal hearts.
Chest x-ray usually demonstrates a globe-shaped heart and is useful to calculate the cardiothoracic ratio which is correlated to the prognosis.
Cardiac catheterization is seldom necessary, unless the patient responds to criteria for preoperative coronary arteries angiography.
5.5 Surgical Techniques
Surgical restoration of the tricuspid valve in patients with EA was developed by Hunter, Lillehei, and Hardy [21–23], and since then, a wide number of surgical techniques have been reported in the literature. The pioneering first surgical attempts aimed to restore TV continence. Later on, the focus moved to restore the valve to the true annulus, to remodel the annulus, and to plicate the atrialized right ventricle. Even more recently many techniques consider to leave the valve to “play where it lies,” and the atrialized right ventricle is not always considered for plication.
In EA, the posterior and septal leaflets are severely compromised, and the anterior leaflet is only able to reach the ventricular septum; thus, coaptation takes place between anterior leaflet and the septum. Indeed, the size and integrity of the anterior leaflet are essential to obtain a good surgical result and are the main key factors in most of the used surgical techniques.
Anterior leaflet mobilization has been introduced by Carpentier et al. [9]. One critical point is whether the anterior leaflet has a free leading edge. In some cases the anterior leaflet has hyphenated attachments or, worst, a linear attachment. In the last case repair is difficult and could be suboptimal.
Presumably EA has such a broad spectrum, including patients with different ages and with different degrees of severity, that surgeons have to focus on different techniques in their surgical armamentarium to overcome the difficulties in dealing with different patients and different tricuspid valve anomalies.
5.6 Indications
Indications to TV surgery for EA have changed over the decades because we assisted to a substantial reduction of the operative mortality and to an increase in repair capabilities. Thus, the actuarial trend is to forward patients to surgery earlier than two decades ago.
In children and young adults presenting in good conditions, without physical limitations, there is no evidence for a “prophylactic surgery” [24] which might have the undesirable result of a valve replacement and to its following sequelae.
Generally, surgery is deemed necessary when symptoms appear. The most common symptom is shortness of breath which could emerge in adulthood, shortly accompanied by reduction in exercise tolerance, cyanosis (intended as mixed oxygen saturation lower than 90 %), arrhythmias, and possibly paradoxical embolism. Sometimes it is not clear from the patient understand the real degree of the impairment of functional capacity. A stress test with oxygen consumption is useful to validate the functional capacity from an objective point of view, and when necessary, it has to be repeated to evaluate the deterioration trend. Supraventricular tachycardias are treated first by beta-blockers and ablation. Surgery will be necessary when there is a failure of these treatments and/or when the physical disability is increasing.
If on the one hand, there is common agreement in recommending a surgical correction in symptomatic patients with EA, more difficult is to delineate surgical indications in asymptomatic patients. Once surviving the neonatal period, patients with EA could have an asymptomatic course and EA allows an acceptable quality of life. Asymptomatic patients have indications to surgery when there is an increase in the size of RV as evidenced by chest x-ray (cardiothoracic ratio greater than 0.65). RV dilation, with a cardiothoracic ratio greater than 0.65, is associated with an increased risk of death [25, 26] either as consequence of the natural history, with an increased risk of sudden death, than during surgical treatment.
This suggests that an earlier indication to surgery could finally lead to the improvement of the long-term outcome [19, 27, 28]. Nevertheless, early age at operation is a risk factor for increased reoperation rate [27, 29], and in case of valve replacement, this exposes the patient to the need for further reoperation for valve replacement.
Moreover, surgery is indicated in asymptomatic patients when max VO2 is not normal [24]. Among different studies it is evident that the reduction of functional class after TV repair is accompanied by increased functional capacity. An increase in the peak oxygen uptake and ventilatory efficiency has been reported. Indeed, abolishing of the TV regurgitation results in a clinical benefit evaluable with an exercise testing [30]. Finally, surgery for EA is indicated when a paradoxical motion of the ventricular septum is shown on echocardiography, discovering an evolution of the LV toward dysfunction.
The operation for EA has been proved to positively affect the LV function [14, 31], and the authors suggest that the decreased LV function should be an indication to TV surgery despite a higher early mortality rate when compared to patients with normal LV function.
A postoperative increase in LV ejection fraction after TV repair has been reported, with a significant increase from 56 ± 10 to 68 ± 8 % [32].
5.7 General Consideration on Surgery
Monitoring should include a central venous pressure line both in a jugular vein and in the femoral vein [33]. Transesophageal echocardiographic probe should be inserted before starting the operation.
The surgical approach is through a median sternotomy. A pericardial patch is harvested prior to infusion of heparin, because it can be useful to augment the anterior leaflet size. The heart is then inspected with a careful attention to the diaframmatic portion of the RV which is usually atrialized. A bulging area of large dimension is predictive of a severe type of malformation. After heparinization, the arterial cannula is positioned in the aorta, while the two venous cannulas are placed in the superior and inferior caval veins. When a bidirectional cavopulmonary anastomosis (BCPA) is scheduled, the superior vena cava has to be dissected free, and the pulmonary artery pressure should be checked before starting the cardiopulmonary bypass (CPB). CPB is started with a mild degree of cooling temperature. After CPB is started and when the heart is empty, the bulging area corresponding to the atrialized right ventricle becomes introflected and its thin consistency becomes evident. The aorta is clamped and the cardioplegic solution is infused. The pulmonary artery can be included in the cross clamp. This will facilitate water testing of the repaired tricuspid valve at the end of the surgical procedure. The surgical procedure could be done on a beating heart only when a PFO or an ASD is absent but CPB remains more comfortable. The right atrium should be opened with a longitudinal incision parallel to the atrioventricular groove with careful attention to the right coronary artery which could lie on the right atrial wall. The left ventricle is vented with a sucker line in the right upper pulmonary vein or directly through the interatrial communication. After repair/replacement of the valve, the ASD is closed directly or using a pericardial patch. The left-sided chambers are de-aired, and the clamp is removed with suction line on the aortic root. The right atrium is then sutured. When a large atrium is present, a part of the right atrial wall with an oval shape could be removed.
If a fenestrated patch is used, the right atrium has to be closed before de-clamping. In this situation, once the atrium is closed, the caval veins are unsnared for de-airing, and then the clamp on the aorta is removed with a suction line in the aortic root. At this time, BCPA has to be performed when scheduled. Weaning of bypass is critical. Sinus rhythm or sequential pacing is mandatory. In many cases, the RV has some degree of dysfunction; thus, the bypass flow has to be reduced very progressively, and the central venous pressure should not exceed 5–10 mmHg. An increase in pulmonary arteries resistances could finally lead to RV distension which has to be avoided. Thus, end expiratory positive pressure should be avoided and nitric oxide could be routinely used [24]; in addition, a small degree of hyperventilation could be helpful. Adrenaline could be used at low doses, 0.01 γ/kg/min, up to 0.05 γ/kg/min. If it is necessary a higher dosage of inotropes to maintain a valuable hemodynamic, then a total assistance, has to be considered. An association between milrinone and adrenaline could be helpful unless low systemic pressure is present. In this case milrinone has to be suspended. After the first 12 postoperative hours, if the hemodynamic is stable, adrenaline infusion could be progressively reduced and milrinone is started or maintained. Amiodarone or lidocaine could be used to control ventricular and supraventricular tachyarrhythmias.
The bypass is stopped with low central venous pressure and is filled progressively. When the filling of the left ventricle induces increase in the central venous pressure (greater than 15 mmHg) and RV dilatation without any effect on arterial pressure (systemic pressure lower than 70–80 mmHg and LA pressure lower than 10 mmHg), the CPB has to be restarted and a BCPA is performed [33].
When the left ventricle is dysfunctioning, the use of an intra-aortic balloon counterpulsation has been described [34]. In our experience ECMO assistance is preferred before the onset of acidosis.
5.8 Repairing Techniques
5.8.1 Mobilization of the Anterior Leaflet and Longitudinal Plication of the Atrialized RV
The technique has been used since the 1980s, is well standardized, and aims to restoring the functionality of both the right ventricle and the tricuspid valve. The main surgical points are an association of mobilization of the anterior leaflet and longitudinal plication of the atrialized RV (aRV) [24, 32, 33, 35]. This repair could be associated with a BCPA in order to reduce the preload of the RV and increase the preload of the left ventricle. Despite that its use is controversial, it has been demonstrated to be useful in severe cases [36] and is more and more often used [34].
Echocardiography is a guide to the surgical choice, and anatomical details have to be evaluated before proceeding to repair. A very severe anomaly with the so-called ventricular sac, type D EA, is an indication to TV replacement.
Once the right atrium is opened and the valve has been examined, the subvalvular apparatus of the anterior leaflet is inspected. The portion closed to the anterior commissure is usually mobile. The leaflet is unfolded by pulling it to the center of the orifice with the forceps, and then an incision is created with a small blade at the anteroseptal commissure and continued with scissors to the posteroanterior commissure (Fig. 5.7). Care has to be taken not to injure the right coronary artery and to leave a small rime of leaflet tissue at the level of the hinge point. A small retractor is inserted in the RV, and the leaflet is pulled toward the right lung in order to better visualize the bands causing tethering of the anterior leaflet. The ventricular aspect of the anterior leaflet is evaluated, and all the muscular attachments and fibrous bands are divided from the base toward the apex (Fig. 5.8). When there is a linear attachment, it is necessary to fenestrate the interchordal spaces to allow right ventricle filling and avoid TV stenosis. The goal of the mobilization is to obtain a free motion of the leaflet. Care has to be taken to avoid dissection of the primary chords, thus avoiding a possible leaflet flail. It is necessary to take care not to perforate the RV when the leaflet is adherent to the endocardium and to avoid perforation of the leaflet itself. Perforations of the upper portion of the leaflet will result in a regurgitant jet and need to be repaired with single stitches. Some patients present a direct attachment of the leaflet to the moderator band without chords. In this case the section of this muscular structure increases leaflet motion. When mobilizing the anterior leaflet, an anterolateral papillary muscle included into the RV wall can be individualized.
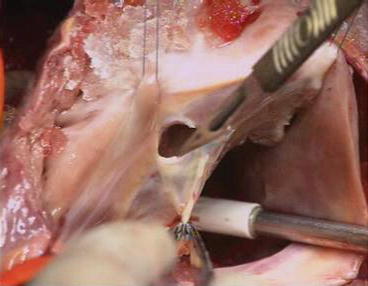
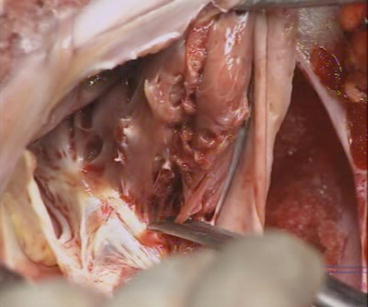

Fig. 5.7
Detachment of the anterior leaflet. The anterior leaflet is unfolded and incised parallel to the AV junction with a scalpel. The incision is extended to both sides, toward the anterior and posterior leaflets

Fig. 5.8
Mobilization of the anterior leaflet. With scissors, the muscular adhesions are divided from the annulus to the apex of the RV. Perforation of the RV ventricular wall can occur, there is no coronary artery in this area, and the suture is performed from inside and/or outside of the RV. Perforations of the leaflet tissue are sutured with a 7/0 Prolene. A perforation close to the posterior leaflet and to the free edge of this leaflet is left intact without any consequence
Once the anterior leaflet anomalies have been addressed, the atrialized chamber is plicated. The chamber has a triangular shape having the summit at the RV apex and the basis on the anatomical annulus. The posterior side of the triangle is the attachment of the aRV to the ventricular septum, and the anterior side corresponds to the adhesion of the anterior and posterior leaflets on the RV wall. The TV annulus is reduced according to the expected value of normal TV size [37], introducing a Hegar dilator into the valve orifice. A suture is passed in the annulus at the level of the anteroposterior commissure and in front of the coronary sinus according to the Hegar size. Stitch pulling helps in evidencing the area which needs plication. Afterward a running 4/0 suture is started from the summit and extended longitudinally till the anatomical annulus. The suture has to be passed on the endocardial layer, avoiding wall perforation. A further suture on the atrial side is needed to exclude the blind cavity which results from the aRV plication. Differently from the transverse plication, the longitudinal one reduces greatly the TV annulus. The epicardial surface of the RV is then carefully inspected to ascertain that any coronary arteries have been injured.
The freed anterior leaflet is then sutured on the new annulus after a clockwise rotation, using a 6/0 or 7/0 Prolene running suture. The clockwise rotation allows the leaflet to cover the annulus till the point facing the coronary sinus. Indeed the suture stops at the coronary sinus level.
The valve competence is tested with a saline injection in the RV chamber (Fig. 5.9). If a residual regurgitant jet still exists, a further reduction of the annulus could be performed using additional stitches on the posterior annulus. In adults a prosthetic ring could be implanted. In this case we use a Carpentier prosthetic ring anchored with 3/0 U-shaped sutures. The appropriate ring must cover the anterior leaflet in size since the anterior leaflet is the only closing element of the orifice.
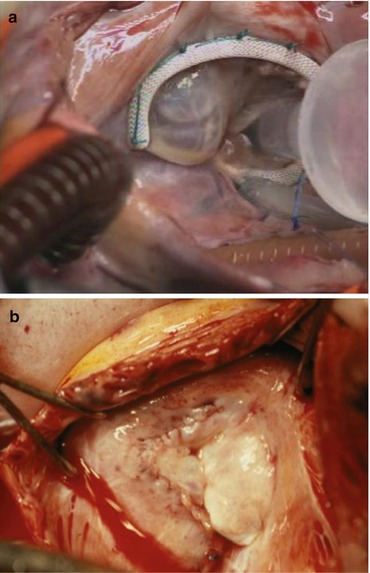

Fig. 5.9
Final result. (a) Control, saline is injected in the RV. A good result is obtained when the anterior leaflet is coapting along the ventricular septum. A prosthetic ring was used in this adult case. (b) Final aspect in a child
Eventually, the residual jet is caused by a persistent restriction of the anterior leaflet which does not reach the septum causing a lack of coaptation. This is the reason for an extensive mobilization which is essential for a good result. Extension of the anterior leaflet has been described in order to increase the size of the leaflet and to use it as a sail [32, 38] or when the anterior leaflet shows a tension at the end of its mobilization. Augmentation is usually performed with fresh autologous pericardium tailored on an oval shape and as big as the anterior leaflet itself. The patch is inserted after anterior leaflet detachment and sutured with a Prolene running suture.
Using this technique, by detaching the anterior leaflet from the annulus and plicating the RV, most of the accessory pathways located are divided. This is usually efficient to reduce arrhythmia from atrial origin [39]. Furthermore, a successful repair with reduction of atrial distension has a known antiarrhythmic effect.
Atrial septal defect is closed either with a direct suture or using a patch except in severe decrease of the RV contractility. In this case it could be left open or reduced using an autologous fenestrated patch at the expense of a certain degree of cyanosis.
When necessary, BCPS is performed.
5.9 The Cone Technique
The cone reconstruction of the TV valve [40] aims at using all the leaflet tissue to reconstruct the valve. At the end of the repair, the orifice is surrounded by leaflet tissue. The new valve is relocated at the anatomical annulus (Fig. 5.10).
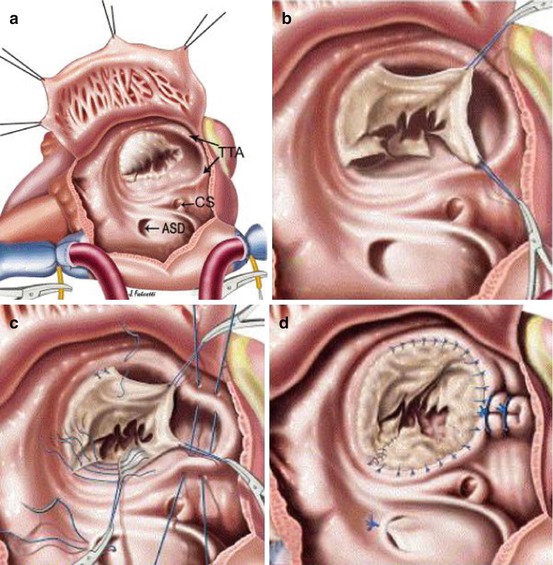

Fig. 5.10
The cone technique. (a) Aspect of the lesions. (b) Detachment of the leaflet tissue. (c) Rotation and suture. (d) Plication of the annulus
The anterior leaflet is detached using a blade, and the incision is extended using scissors on a clockwise direction. The dissection continues to the posterior leaflet which has to be detached and surgically delaminated from the RV wall. The two leaflets have to be fully mobilized. The septal leaflet is detached from the septum and mobilized with a scissor toward the anteroseptal commissure. After mobilization of the three leaflets, the cutted edge of the posterior leaflet is sutured to the cutted edge of the septal leaflet. Atrialized right ventricle is plicated longitudinally. Further plication of the posterior annulus is needed to create a match between the cone obtained by the three leaflets and the new annulus. Leaflet tissue is finally sutured to the new annulus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree