The late worsening of nonsevere tricuspid regurgitation (TR) after mitral valve surgery is a relevant clinical problem that can lead to high-risk reoperation. Although tricuspid annulus (TA) dilatation has been proposed for prophylactic annuloplasty to prevent TR worsening, prospective data in degenerative mitral regurgitation (MR) are lacking. The aim of this prospective cohort study was to evaluate TA dimension to predict TR progression after valve repair for degenerative MR. Clinical and echocardiographic evaluation of 706 patients with degenerative MR and no significant TR was obtained preoperatively and at follow-up after isolated mitral valve repair. Together with standard cardiac chamber and valve analysis, 3-dimensional (3D) transesophageal echocardiography was performed to evaluate TA, including the anteroposterior and septolateral diameters. After a mean follow-up of 24 ± 15 months (range 6 to 60), 2 patients died while 14 developed severe MR. Compared with preoperative values, TR decreased (≤1 degree) in 227 patients, was unchanged in 437, and increased (≥1 degree) in 39 patients, with the development of significant TR (3 to 4 degree) in 3 patients. Receiver-operating characteristic curve analysis did not identify significant TA values predicting postoperative TR worsening. On multivariate regression analysis, recurrent MR and pulmonary hypertension at follow-up emerged as significant positive predictors of TR progression. Newly developed significant TR is a rare event after successful repair of degenerative MR. Although more accurate than conventional 2D measurement, 3D analysis of TA does not predict early to midterm subsequent TR progression.
Tricuspid regurgitation (TR) can represent an important cause of morbidity and mortality in patients after mitral valve (MV) surgery. It is generally considered that severe functional TR needs to be treated at the time of the MV intervention, whereas much debate surrounds the optimal management of less-than-severe TR. In recent years, many studies have reported the development of significant late TR despite successful MV surgery, especially in subjects presenting with a dilated tricuspid annulus (TA). Tricuspid annular measurement approaches varied significantly, ranging from qualitative estimation and 2-dimensional transthoracic echocardiography (2D-TTE) to intraoperative sizing by the surgeon at arrested heart. At present, there is no conclusive evidence regarding the appropriateness of preoperative TA evaluation and the planning of a surgical strategy in the absence of significant TR. Furthermore, recent retrospective studies report that TA dilatation and TR progression may be less significant in patients with degenerative mitral regurgitation (MR) compared with other groups (e.g., rheumatic MR and functional MR). In the present study, we prospectively evaluated the predictive value of TA size as assessed by real-time 3D transesophageal echocardiography (3D-TEE) for the development of significant TR in a large cohort of patients undergoing MV repair for degenerative MR.
Methods
In this prospective cohort study, consecutive patients were assessed using 2D-TTE and 2D-/3D-TEE before MV repair for degenerative MR (prolapse or flail leaflet) at our department from January 2008 to December 2012. Patients with endocarditis, severe left ventricular dysfunction (ejection fraction <35%), and those requiring diuretic medication were excluded, together with those with poor-quality 3D-TEE images, and those without at least 6-month follow-up after surgery. A total of 106 patients with significant TR (3/4+ degree) undergoing tricuspid valve repair at the time of MV surgery were excluded, resulting in a final population of 706 prospectively enrolled subjects. The study was approved by the local ethics committee and complied with the Declaration of Helsinki. All subjects gave written informed consent.
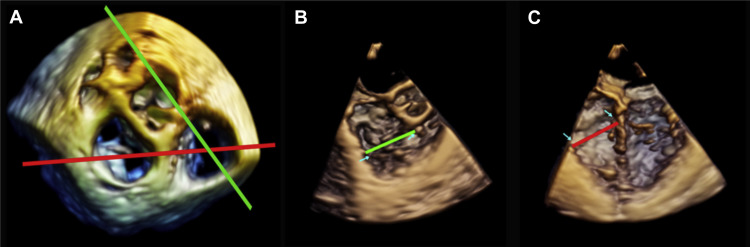
All subjects underwent 2D-TTE and 2D-/3D-TEE examination the day before surgery. We performed standard 2D-TTE with an iE33 (Philips Ultrasound, Andover, Massachusetts) connected to an S5 probe. All images were acquired and stored in digital cine loops, and subsequent standard cardiac chamber and valve analysis was carried out. MR severity was graded with a 4-point scale using the conventional multiparametric approach. TR was graded according to American Society of Echocardiography and European Association of Cardiovascular Imaging recommendations using the following parameters: vena contracta width, effective regurgitant orifice area, and planimetric jet area. Doppler parameters were obtained from the apical view, permitting best visualization of the TR jet. All TR parameters were assessed during inspiration and expiration, and the results were averaged; TR was graded on a 4-point scale using current parameters : grade 0: no TR; grade 1 (mild) if jet area was <5 cm 2 ; grade 2 (moderate) if no parameter reached cutoff severity and jet area was 5 to 10 cm 2 ; grade 3 (moderate to severe) if 1 or 2 parameters reached cutoff severity; grade 4 (severe) if TR fulfilled the following cutoffs: vena contracta width >7 mm, effective regurgitant orifice >0.4 cm 2 , and jet area >10 cm 2 . Systolic flow reversal in the hepatic veins and triangular continuous wave regurgitant jet signal were used as supporting signs of severe TR. All patients with more than moderate (3 to 4+) preoperative TR were excluded. Standard TA diameter (4-chamber view), systolic tenting of tricuspid valve leaflet, and right ventricular (RV) transverse and longitudinal diameters were measured at TTE using an optimized 4-chamber view, both at end systole and end diastole. RV function was evaluated using tricuspid annular plane systolic excursion and peak systolic tissue Doppler imaging. Peak pulmonary artery pressure was estimated from the velocity of the tricuspid regurgitant jet. After transthoracic examination, 2D- and 3D-TEE were performed using an X7-2t TEE probe. Standard 2D and 3D views were adopted to assess MV as previously described. TA diameters were measured from 3D-TEE volume zoom-mode data sets, including the whole TA and the aortic valve, prospectively acquired using electrocardiographic gating and suspended respiration. Four-beat acquisition and frame rates of at least 20 fps were considered acceptable; care was taken to optimize image quality without artifacts or dropouts and to ensure that the entire TA throughout the cardiac cycle was included. In patients with atrial fibrillation, images were acquired using a specific volume protocol, avoiding stitch artifacts. Real-time 3D data sets were subsequently transferred to a Cardio-View workstation (TomTec Imaging Systems, Munich, Germany) for offline analysis and TA measurement. To assess TA diameters, the 3 standard planes (sagittal, coronal, and transverse) were displayed simultaneously, with the rendered 3D image in the bottom right-hand corner. Anatomical views were used to perform TA measurement ( Figure 1 ). Planes were oriented so that the anteroseptal commissure was the most anterior aspect of the annulus and with the anteroposterior (AP) and septolateral (SL) planes intercepting in the middle of the orifice. The 2 measurement planes were then guided by the 3D rendered image, with the AP plane crossing the middle of the orifice, the anteroseptal commissural zone and the aorta in short axis, and the SL plane crossing the middle of the orifice, the anterior and septal leaflets, the RV apex and the interventricular septum below the left ventricular outflow and the aorta. SL and AP diameters were measured both at end systole and at end diastole. TA eccentricity ratio was calculated as the ratio between AP and SL diameters. All measurements were repeated 3 times and then averaged. Complete data concerning time of extracorporeal circulation, time of aortic clamping, duration of stay in intensive care, postoperative renal failure, and early (<48 hours) left or RV dysfunction were collected from hospital records. All patients underwent routine TTE before discharge. Follow-up visits included clinical assessment, electrocardiogram and standard TTE. Evaluation of echocardiographic parameters was carried out in the absence of diuretic medication or after 48 hours. Causes of death were verified by telephone contact with relatives, the general practitioner, or hospital certification.
Continuous data are expressed as means ± SD or when appropriate as medians (25 to 75 percentiles); discrete data are reported as frequencies and percentages. A p value <0.05 was considered statistically significant. Data were analyzed using SPSS 19 (IBM Corp., Armonk, New York). The Shapiro–Wilk test was used to verify normal distribution of continuous variables. Normal distributions were compared using the t test for independent samples or one-way analysis of variance (ANOVA) when appropriate. Non-Gaussian distributions were compared using the Mann–Whitney U test (2-group comparisons), or the one-way ANOVA with the Kruskal–Wallis test (3 or more groups). Discrete variables were analyzed using the chi-square test. TR severity at baseline, predischarge, and follow-up were compared using repeated-measures ANOVA, treating strata of duration of follow-up as a between-subjects factor, and using Tukey’s post hoc test for pairwise comparisons between strata. Spearman’s rho was used for correlation analysis, including discrete variables. Univariate linear regression analysis was performed on each relevant independent variable using the worsening in TR severity (≥+) as the dependent variable. Preoperative variables found to be significant predictors at univariate analysis (p <0.05) were then inserted into a forward multivariate stepwise linear regression model. A descriptive multivariate model, including some postoperative variables, was also analyzed. Receiver-operating characteristic curve analysis was performed to test TA measurement cut-off values to predict postoperative TR worsening. Reproducibility of TA measurements was assessed in a randomly selected subgroup of 20 patients of the study population as interobserver and intraobserver and test-retest variability.
Results
Table 1 summarizes clinical and echocardiographic characteristics of the study population. Figure 2 shows end-systolic SL and AP diameters assessed by 3D-TEE. Using a cutoff ≥40 mm or ≥21 mm/m 2 , 49 patients (6.9%) and 65 patients (9.2%) had preoperative SL dilatation, respectively. Three hundred fifty-three patients (50%) had preoperative AP ≥40 mm. Six hundred forty-three subjects with 0/1+ TR had TA diameters of 32.7 ± 4.4 (SL) and 39.8 ± 5.1 (AP), respectively; the remaining 63 patients with 2+ TR had TA diameters of 34.6 ± 4.7 (SL) and 42.5 ± 5.3 (AP). The difference between these 2 subgroups was statistically significant for both AP and SL diameters (p = 0.001 for AP, p = 0.004 for SL). No significant differences were found in terms of TA diameter between patients with atrial fibrillation (n = 53, 7.6%) and those in sinus rhythm (41.2 ± 5.8 vs 39.9 ± 5.2, p = 0.12 for AP; 34.0 ± 4.2 vs 32.8 ± 4.5, p = 0.07 for SL). No statistically significant differences between systolic and diastolic TA diameters were observed. No patients showed systolic tenting of tricuspid valve. Intraobserver and interobserver variability and test-retest variability values were 0.8 ± 0.5, 0.7 ± 0.4, and 0.8 ± 0.7, respectively.
| Parameters | Baseline values (n=706) |
|---|---|
| Age (years) | 57.1 ± 13.7 |
| Male | 523 (74.1%) |
| Female | 183 (25.9%) |
| Body Surface Area (m 2 ) | 1.86 ± 0.20 |
| NYHA class | |
| I | 307 (43.5%) |
| II | 395 (55.9%) |
| III | 4 (0.6%) |
| Arterial Hypertension | 141(20%) |
| Atrial Fibrillation | 53 (7.6%) |
| Left Ventricular End-Diastolic Diameter (mm) | 59.0 ± 6.7 |
| Left Ventricular End-Systolic Diameter (mm) | 34.7 ± 5.9 |
| Left Ventricular End-Systolic Diameter ≥ 40 mm | 180 (25.4 %) |
| Left Ventricular Ejection Fraction (%) | 62.6 ± 7.0 |
| Left Ventricular Ejection Fraction <45% | 10(1.4%) |
| Mitral Regurgitation Etioloy | |
| Myxomatous | 546 (77%) |
| Fibroelastic Deficiency | 160 (33%) |
| MR severity | |
| 0/1+ | 0 |
| 2+ | 0 |
| 3/4+ | 706 (100%) |
| Systolic Pulmonary Artery Pressure (mmHg) | 30 (26-34) |
| Concomitant procedures | |
| Atrial Fibrillation ablation | 37 (5.2%) |
| Coronary bypass | 27 (3.8%) |
| Patent Foramen Ovale closure | 17 (2.4%) |
| Tricuspid annulus Antero-Posterior diameter (mm) | |
| Diastole | 42.2 ± 5.2 |
| Systole | 40.0 ± 5.2 |
| Tricuspid annulus Septo-Lateral diameter (mm) | |
| Diastole | 35.2 ± 4.4 |
| Systole | 32.9 ± 4.5 |
| Tricuspid annulus Antero-Posterior diameter/m 2 | |
| Systole | 21.7 ± 3.2 |
| Tricuspid annulus Septo-Lateral diameter/m 2 | |
| Systole | 17.8 ± 2.5 |
| Tricuspid annulus eccentricity ratio (Antero-Posterior/Septo-Lateral) | |
| Diastole | 1.20 ± 0.14 |
| Systole | 1.22 ± 0.15 |
| Right Ventricular basal diameter (mm) | |
| Diastole | 35.0 ± 4.7 |
| Systole | 29.9 ± 4.3 |
| Right Ventricular longitudinal diameter (mm) | |
| Diastole | 59.1 ± 6.8 |
| Systole | 52.1 ± 6.4 |
| Tricuspid annular plane systolic excursion (mm) | 20.0 ± 1.4 |
| Tricuspid Annular Systolic Tissue Doppler Imaging (cm/sec) | 12.6 ± 4.5 |
| Tricuspid Regurgitation severity | |
| 0-1/4 | 643 (91.6%) |
| 2/4 | 63 (8.4%) |
| 3-4/4 | 0 |
All patients underwent surgical MV repair with median sternotomy (n = 656), or minithoracotomy (n = 50). Concomitant procedures included coronary bypass, atrial fibrillation surgery, and patent foramen ovale closure. There were no in-hospital deaths. Early postoperative complications included: low-output syndrome (11%), left ventricular dysfunction (17%), RV dysfunction (16%), renal failure (11%), stroke (0.4%), need for surgical revision for bleeding/tamponade (1.4%), and early redo mitral repair (1.4%). One patient underwent MV replacement due to early repair failure and was excluded from subsequent analyses. Predischarge echocardiography demonstrated residual mild-to-moderate (2+) MR in 16 patients (2.3%), with no or trace MR in the remaining patients (98%). TR severity decreased at predischarge echocardiography compared with preoperative evaluation (from 1.04 ± 0.40 to 0.66 ± 0.53). After a mean follow-up of 24 months (±15; median 23; range 6 to 60 months), 2 patients died (1 sudden death and 1 noncardiac death), and 14 developed severe MR due to repair failure. Table 2 reports TR changes based on time to ≥6-month last visit (<24 months: n = 396; 24 to 48 months: n = 145; >48 months: n = 69). Three patients (0.4%) who developed new significant TR during follow-up were managed medically, without subsequent repair surgery. Five more patients showed an increase of 2 grades (from 0+ to 2+) in TR severity, whereas 31 showed an increase of one grade (from 0 to 1+ or from 1+ to 2+). Clinical and echocardiographic characteristics of the study population were comparable to those of the excluded patients with incomplete follow-up (<6 months). Table 2 reports the comparison between patients with improving, stable, or worsening TR at follow-up. Apart from lower left atrial volume and systolic pulmonary artery pressure (sPAp) values, patients with worsening TR at follow-up did not show significant differences concerning preoperative echocardiographic parameters. Figure 3 reports subgroup analysis of TR changes from baseline to last follow-up, showing significant differences only in patients with recurrent MR and sPAP at follow-up. Figure 4 shows no significant changes in TR severity at follow-up among different quartiles of TA diameters, both using raw and indexed values. Nonsignificant changes in TR were also observed when patients were stratified according to ≥40 mm or ≥21 mm/m 2 SL diameter. In addition, receiver-operating characteristic curve did not identify any TA measurement cutoff as a predictor of TR worsening at follow-up. At multivariate regression ( Table 3 ), recurrent significant MR and sPAP at follow-up were associated with postoperative TR worsening, whereas in the predictive model TA dilatation indexes or preoperative 1 to 2+ TR did not affect TR progression, at least in this time window. The relation between TR changes and recurrent MR and sPAP at follow-up is shown in Figure 5 .
| Variables | Tricuspid Regurgitation at Follow-up | |||
|---|---|---|---|---|
| Decreased (n = 227 ) | Unchanged (n = 437) | Worsening (n = 39) | P ∗ | |
| Left Ventricular End-Diastolic Diameter (mm) | 59.9 ± 6.6 | 58.7 ± 6.8 | 57.2 ± 6.2 | 0.43 |
| Left Ventricular End-Systolic Diameter (mm) | 35.1 ±6.0 | 34.6 ± 5.8 | 34.6 ± 6.0 | 0.57 |
| Left Ventricular Ejection Fraction (%) | 61.6 ± 7.5 | 62.9 ± 7.0 | 64.4 ± 7.1 | 0.07 |
| Left Atrial volume (ml) | 84 (59-115) | 77 (60-100) | 64 (45-90) | 0.01 ∗ |
| Extra Corporeal Circulation time (min) | 79 (65-98) | 76 (65-95) | 69(60-90) | 0.28 |
| Systolic Pulmonary Artery Pressure (mmHg) | 32 (30-40) | 30 (29-37) | 30 (25-34) | 0.04 ∗ |
| Tricuspid Annular Antero-Posterior Diameter (mm) | ||||
| Diastole | 43.3 ± 4.8 | 41.6 ± 5.3 | 42.1 ± 4.8 | 0.87 |
| Systole | 41.0 ± 4.8 | 39.5 ± 5.4 | 39.8 ± 4.8 | 0.95 |
| Tricuspid Annular Septo-Lateral Diameter (mm) | ||||
| Diastole | 36.1 ± 4.8 | 34.6 ± 4.5 | 34.8 ± 3.0 | 0.97 |
| Systole | 33.8 ± 4.4 | 32.4 ± 4.5 | 32.5 ± 2.9 | 0.99 |
| Tricuspid Annular Antero-Posterior Diameter/Body Surface Area (mm/m 2 ) | ||||
| Systole | 22.0 ± 3.1 | 21.5 ± 3.3 | 21.9 ± 3.0 | 0.77 |
| Tricuspid Annular Septo-Lateral Diameter/Body Surface Area (mm/m 2 ) | ||||
| Systole | 18.1 ± 2.5 | 17.6 ± 2.5 | 18.0 ± 2.1 | 0.66 |
| Tricuspid Annular Eccentricity Ratio (Antero-Posterior/Septo-Lateral diameters) | ||||
| Diastole | 1.20 ± 0.14 | 1.21 ± 0,14 | 1.21 ±0.13 | 0.99 |
| Systole | 1.22 ± 0.15 | 1.22 ± 0,15 | 1.22 ±0.14 | 0.97 |
| Right V basal diameter (mm) | ||||
| Diastole | 35.3 ± 4.8 | 34.8 ± 4.7 | 35.1 ± 4.0 | 0.94 |
| Systole | 30.1 ± 4.2 | 29.7 ± 4.2 | 29.9 ± 3.5 | 0.97 |
| Right Ventricular Longitudinal Diameter (mm) | ||||
| Diastole | 59.8 ± 7.1 | 58.9 ± 6.6 | 57.5 ± 6.0 | 0.47 |
| Systole | 52.3 ± 6.3 | 51.9 ± 6.6 | 51.0 ± 5.6 | 0.68 |
| Tricuspid Annular Plane Systolic Excursion (mm) | 19.9 ± 1.6 | 20.0 ± 1,3 | 19.5 ± 1.0 | 0.15 |
| Tricuspid Annular-SystolicTissueDoppler Imaging (cm/sec) | 12.8 ± 4.5 | 12.8 ± 4,6 | 12.5 ± 4.5 | 0.15 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


