CHAPTER 125 Transposition of the Great Arteries
Simple and Complex Forms
HISTORY
Transposition of the great arteries (TGA) was first recognized and described more than 2 centuries ago. Although reference was made to malposition of the aorta and pulmonary artery by Steno in 1672 and Morgagni in 1761, the anatomic description of TGA is credited to Baillie in 1797.1 It was Farre in 1814 who introduced the term transposition of aorta and pulmonary artery, meaning that the aorta and pulmonary artery are displaced across the ventricular septum.2 An attempt to classify the various types of transposition was reported by Von Rokitansky, and the first clinical recognition of TGA in life was noted by Fanconi in 1932.3,4 In 1938, Taussig described the pathologic anatomy and hemodynamic and clinical characteristics of the cardiac defect.5
With the development of cardiac surgery in the 1950s, effective therapy for this disease has become available only relatively recently. Initial treatments were palliative and included atrial septectomy, first described by Blalock and Hanlon.6 The first successful attempts to reroute the circulations were at the atrial level. These were followed later by successful arterial-level repair and correction of TGA and ventricular septal defect (VSD). These developments are further described in the following section.
EPIDEMIOLOGY
Today, TGA is known to be relatively common, accounting for 9.9% of infants with congenital heart disease, or 0.206 per 1000 live births, in one New England study.7 There is a 2:1 male-to-female ratio that increases to 3.3:1 when the ventricular septum is intact.8 In complex forms of transposition, gender predominance has not been noted. If TGA is not treated, 90% of children with D-transposition and intact septum will die by 1 year of age.
CLASSIFICATION AND EMBRYOLOGY
Several theories have been advanced about the morphogenesis of the abnormal relationship between the great arteries and the ventricles in TGA. It has been suggested that the subaortic conus persists and develops during normal looping of the ventricles, whereas the subpulmonary conus undergoes absorption and thus establishes eventual fibrous continuity between the mitral and pulmonary valves, the reverse of the normal situation.9 In the normal heart, the subaortic conus does not grow, and dominant growth of the pulmonary conus forces the pulmonary valve anterior, superior, and to the left. In transposition, differential growth of the subaortic conus pushes the aorta anteriorly and disrupts aortic to mitral valve continuity. If the subpulmonary conus fails to develop, the pulmonary artery will maintain a posterior location, and pulmonary to mitral valve continuity will occur. As a consequence of this relationship, the aortic valve becomes anterior to the pulmonary valve, permitting both semilunar valves to connect with the distal great vessels without the rotation that is hypothesized to occur in normal cardiac development. Because conal development determines rotation of the truncus arteriosus, the great arteries are similar in relationship at the semilunar valves as they are at the arch, resulting in no twist in the great arteries.
Some debate also exists about the relationship between the various conotruncal abnormalities, which also include tetralogy of Fallot and double-outlet right ventricle. Recent evidence suggests that TGA may be unique in that it is not seen in the spectrum of conotruncal abnormalities generated from the most common experimental model of these disorders.10 A new animal model, which is the most reliable to date for producing isolated D-TGA, suggests that TGA may be due to abnormal migration of neural crest cells during a critical phase of outflow tract development.11
ANATOMY
Anatomic variations in TGA are often encountered, increasing the surgical complexity of repair, described later in this chapter. Normal atrial situs occurs in 95% of patients; left-to-right juxtaposition of the atrial appendages signals the presence of other intracardiac anomalies. Although a true ostium secundum atrial septal defect (ASD) is present in 10% to 20% of cases, the majority of atrial communications are through a patent foramen ovale. Right aortic arch is present in 4% of patients with intact ventricular septum and in up to 16% of those with VSD.12 Up to 50% of patients with TGA have an associated VSD, many of which spontaneously close.13 The VSDs are commonly perimembranous, although they may be found anywhere in the ventricular septum. Pulmonary stenosis or atresia, overriding or straddling atrioventricular valves, coarctation of the aorta, and interruption of the aortic arch have all been noted in association with transposition and VSD.
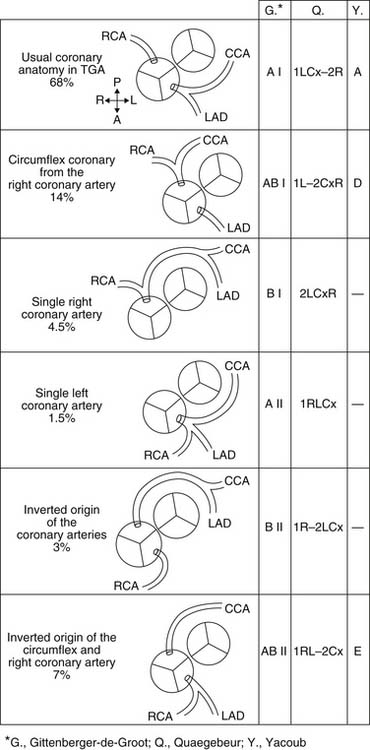
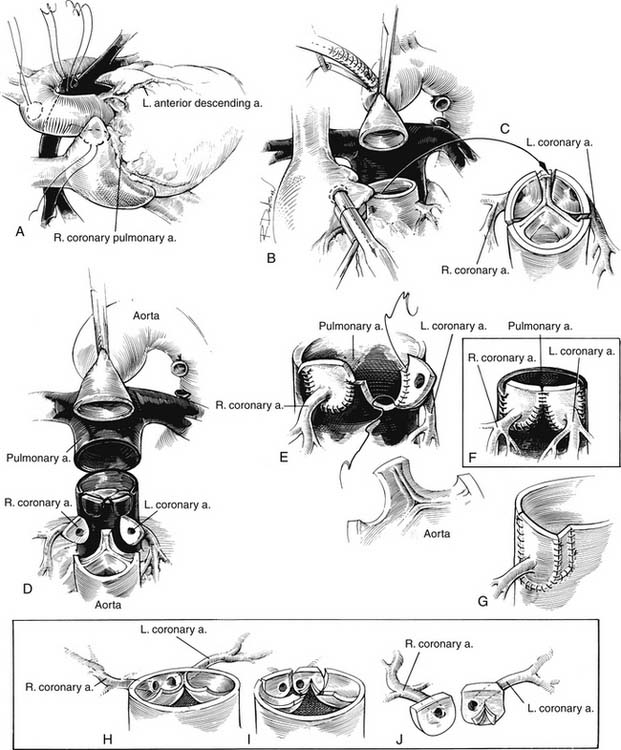
The spatial relationship of the great vessels is quite variable; however, the aorta is most frequently to the right and anterior to the pulmonary artery. In almost all cases, the sinuses of Valsalva and the coronary artery ostia face the corresponding pulmonary arterial sinuses. This situation is favorable for transfer of the coronary arteries with the arterial switch operation, although the origin of a coronary artery from a nonfacing sinus poses a problem for arterial switch in only a small number of patients. Many classification systems have been used to describe the coronary anatomy in TGA. The most widely accepted scheme within the surgical community is the Leiden classification system, which is depicted with the other common systems of classification in Figure 125-1. The most common coronary pattern in D-TGA (68%) consists of the left main coronary arising from the leftward coronary sinus, giving rise to the left anterior descending and circumflex coronary arteries.14 The right coronary arises as a separate ostium from the rightward posterior-facing sinus. On occasion, there is no true circumflex coronary artery, but separate branches arise from the left coronary to supply the corresponding portion of the left ventricle. In up to 20% of cases, the circumflex coronary arises from the right coronary artery off the rightward posterior-facing sinus and passes behind the pulmonary artery. The left anterior descending artery then arises from a separate coronary ostium off the left coronary sinus. More rare coronary patterns involve a single right coronary artery from the rightward posterior sinus (4.5%) or a single left coronary artery from the leftward coronary sinus (1.5%).15 Intramural coronary arteries that proceed in the aortic wall for a distance before exiting to the epicardial surface have been described and commonly occur at the commissural attachment of the semilunar valve.16 Single coronary ostium or separate ostia close together arising from a single sinus have also been described. Abnormal coronary anatomy is more commonly seen in transposition with VSD than in the intact septum variety. Abnormal coronary patterns have also been found to be more common when the relationship of the great arterial trunks is not usual (aorta anterior and to the right).17
CLINICAL FEATURES
Patients with TGA and significant VSD often have higher oxygen saturations by virtue of greater pulmonary blood flow and greater mixing at both atrial and ventricular levels. In children with high pulmonary blood flow, pulmonary resistance may progressively increase throughout infancy. Ferencz18 has observed significant histologic changes in children older than 2 years with TGA, and children as young as 1 month have had intimal fibrosis noted in the pulmonary arteries. This early development of severe pulmonary vascular disease in children with TGA is exacerbated in patients with associated VSD, but early important pulmonary vascular disease can occur in patients with TGA regardless of the presence of a VSD as demonstrated by Ferguson in 1960 and Ferencz in 1966.18,19 The rapidity of development of pulmonary obstructive disease may be related to hypoxemia associated with increased sympathetic activity and excessive pulmonary blood flow.20
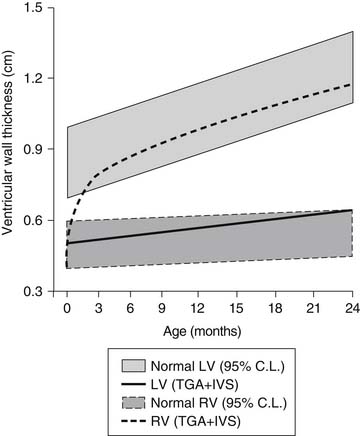
Although neonatal pulmonary vascular resistance is normal in infants with TGA, the resistance falls progressively during the neonatal period with associated changes in the pulmonary and systemic ventricular compliance. Shortly after birth in the normal infant, there is an increase in the left ventricular volume load and pressure load and a decrease in right ventricular volume and pressure load. These physiologic changes result in a rapid increase in the left ventricular myocardial mass.21 This normal development of the left ventricle is lost in infants with D-TGA, in which the left ventricle ejects to the lower resistance pulmonary vascular bed (Fig. 125-2). Thus, the left ventricle does not increase muscle mass relative to the right ventricle, and within a few weeks it loses the ability to maintain adequate cardiac output against significant afterload. This change occurs despite the fact that the left ventricle still maintains a volume load in patients with transposition and intact ventricular septum. However, when a VSD or large PDA is present, both volume and pressure overload of the left ventricle is maintained, and in D-TGA with left ventricular outflow tract obstruction without a VSD, a ventricular pressure load is imposed without a significant volume load. These physiologic changes in the neonatal heart are important for the consideration of surgical approaches because after a few weeks to months of postuterine life, the left ventricle in D-TGA with intact ventricular septum takes on the characteristics and wall thickness of a pulmonary ventricle and may not be adequate to support the systemic circulation.
DIAGNOSIS
Physical Examination
Cardiac examination typically reveals a mildly overactive precordium, and 75% of patients with TGA and intact ventricular septum have a soft systolic murmur. The second heart sound is typically single and loud because of the proximity of the aorta to the anterior chest wall, which may make assessment of pulmonary vascular resistance difficult.20 End-diastolic gallop rhythms are often noted in patients with associated VSD. In the presence of a large PDA, the femoral pulses are often bounding. The liver may be enlarged in patients with a large systemic-to-pulmonary shunt and may be associated with tachypnea, intercostal retractions, and inability to successfully nurse. Children with significant valvular or subvalvular pulmonary stenosis often have a crescendo-decrescendo cardiac murmur along the left sternal border transmitted to the right clavicular area.
PREOPERATIVE MEDICAL MANAGEMENT
Patients who are good anatomic candidates for an arterial switch procedure and have acceptable arterial oxygen saturation are generally referred for early operation if the clinical condition permits. Prostaglandin E1 is usually administered to maintain ductal patency, to increase pulmonary blood flow, and to improve stabilization of patients before early operative repair. Because relative dehydration may decrease the degree of interatrial shunting, volume infusions may improve hemodynamics in infants presenting with TGA and intact septum early in life. A major development in the treatment of TGA occurred in 1966, when Rashkind and Miller reported the use of a balloon catheter technique to enlarge the ASD in patients with TGA, improving early physiologic stability.22 If there is significant instability as evidenced by persistent acidemia or hypoxemia despite conservative measures, the mainstay of management has become a Rashkind balloon atrial septostomy.
In the neonate with TGA, left atrial pressure is usually greater than the right atrial pressure. The pressure in the pulmonary (left) ventricle is dependent on the presence or absence of a VSD, valvular or subvalvular stenosis, age of the patient, and pulmonary vascular resistance. If inadequate interatrial shunting is noted, a Rashkind atrial septostomy is performed.22 A balloon-tipped catheter is passed through the systemic veins, through the right atrium and foramen ovale, and into the left atrium; the balloon is inflated and pulled vigorously across the atrial septum to tear the foramen ovale, improving the admixture of pulmonary and systemic venous blood and tissue oxygen delivery. Although this procedure is usually performed at cardiac catheterization, it may be performed in the intensive care unit with echocardiographic guidance in children who are very unstable. Performance of a Rashkind atrial septostomy results in decompression of the left ventricle and therefore may result in poor left ventricular performance if a subsequent arterial switch procedure is delayed.
SURGICAL MANAGEMENT
Palliative Operations
Initial surgical therapy for TGA involved creation of an ASD with a closed technique to increase the mixing between systemic venous and pulmonary venous circulations by Blalock and Hanlon6 in 1950. Although the early mortality was high with this operative approach, successful creation of an ASD resulted in significant palliation in many of these children. The introduction of the balloon atrial septostomy has essentially eliminated the need for operative atrial septectomy. Infants with associated cardiac abnormalities and a thick atrial septum who are considered for later atrial baffle repair may benefit from the Blalock-Hanlon technique, although the safety of cardiopulmonary bypass has resulted in the common use of open atrial septectomy in these patients.
Atrial Repairs
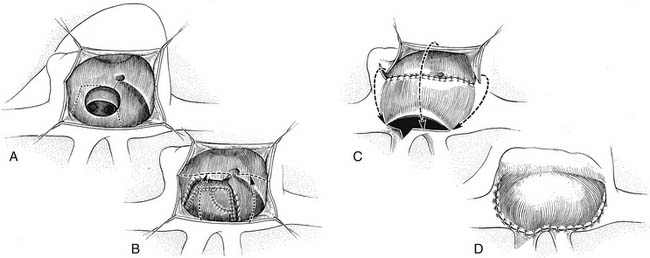
Initial attempts to reverse the transposed vessels in patients with TGA by both Mustard and Bailey and their colleagues were frustrated by their inability to maintain coronary perfusion and by poor function of the anatomic left ventricle.1,23 Thus, initial surgical therapy was directed toward atrial transposition of pulmonary and systemic venous returns. In 1952, Lillehei and Varco24 transferred the right-sided pulmonary veins to the right atrium and connected the inferior vena cava to the left atrium. A successful modification of this technique using an allograft to connect the inferior vena cava to the left atrium was described by Baffes25 in 1956. In 1954, Albert26 suggested the concept of switching the atrial septum so that caval return was directed to the left ventricle and pulmonary venous return to the right ventricle at the atrial level. This atrial switch concept was first successfully accomplished by Senning27 in 1959 by use of an ingenious technique for relocating the walls of the right atrium and the atrial septum. In this operation, pulmonary and systemic venous return was rerouted by incising and realigning the atrial septum over the pulmonary veins and using the free right atrial wall to create a pulmonary venous baffle (Fig. 125-3). The Senning operation was not associated with high survival at first because of its complexity and by the fact that the operation was performed in children between 1 and 2 years of age or with a VSD, and significant pulmonary vascular obstructive disease had already developed in many of these children.
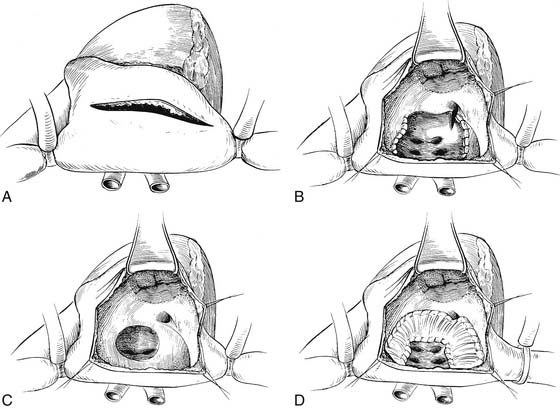
In 1964, Mustard described an alternative procedure for intra-atrial repair, excising the atrial septum and creating a large interatrial baffle of pericardium to redirect pulmonary and systemic venous blood (Fig. 125-4).28 This repair resulted in a larger atrial size than with the Senning operation. In the Mustard operation, creation of a virtual common atrium is necessary with resection of the atrial septum and the ridge of tissue between the superior vena caval entrance and the superior aspect of the atrial septum. Inadequate resection of the atrial septum has resulted in the occurrence of baffle obstruction of the systemic venous return to the heart. Early results with the Mustard operation were markedly improved over the previously reported Senning repairs and reflected the significant number of patients in Toronto who had had successful Blalock-Hanlon atrial septectomies early in life. Because of its technical simplicity, the Mustard operation became the most commonly performed procedure for transposition during the decade of the 1960s, and it became clear that repair in the first few months of life could be accomplished with a low operative mortality and improved results compared with repair at a later age. In 1970, the Senning procedure reemerged as the persistent problems of baffle obstruction and arrhythmias after the Mustard operation became well defined and the ability to use autologous tissue for the atrial reconstruction became a preferred approach. Performance of the Senning operation in infancy after successful balloon atrial septostomy became the preferred technique in many centers.
Outcome after Senning and Mustard Operations
Overall outcomes of these two operations have been good. As techniques improved, the use of either Senning- or Mustard-type repairs in infancy was noted to be associated with a lower incidence of cerebral thrombosis and hypoxic injury due to cyanosis and peripheral emboli from right-to-left shunting. A standard approach developed with balloon atrial septostomy in infancy followed by the elective repair in infants between the ages of 3 and 8 months. Operative mortality in many larger series ranged between 2% and 10% even for patients in early infancy.29–31 Long-term actuarial survival for all patients with TGA undergoing atrial switch procedures is reported to be 88% at 10 years, 76% to 82% at 20 years, and 74% to 77% at 25 years.32 Results are slightly better in isolated TGA compared with TGA associated with VSD or other complicating conditions.
Late systemic (right) ventricular dysfunction occurs in between 5% and 25% of patients. It is the most common cause of late mortality, being the cause of death in approximately 6% of patients in one large series.29 Several challenges exist in observing these patients. The reported incidence of late right ventricular dysfunction is variable. However, if right ventricular function is evaluated objectively, the systemic right ventricle virtually always functions abnormally compared with controls. One of the more recent studies using echocardiographic analysis of right ventricular longitudinal shortening showed that right ventricular function could be judged abnormal in all 20 patients, depending on the parameter evaluated.33 There has also been some difficulty in predicting those patients with measurable right ventricular dysfunction who will progress and require further intervention. It has been suggested that late right ventricular dysfunction may be at least partially due to perfusion defects, which are common in these patients, although the etiology is unclear.34 Another group suggests that marked increase of the right ventricular muscle mass (hypertrophy) in response to systemic afterload may be a contributor.35
Late functional outcome has been reported to be good. One series with good long-term follow-up reported 66% of patients in New York Heart Association (NYHA) class I and 29% in class II.32 Even in the asymptomatic patients, a significant percentage had suboptimal maximal oxygen uptake (MO2) when tested. A similar finding was noted by Ebenroth, who looked at patients an average of 14 years after surgery and found that 75% had normal right ventricular ejection fractions and 84% perceived themselves to be in good health, but only 51% had normal exercise tolerance.32 Varied results from exercise testing in these patients have been reported. One study showed a decrease in the normal cardiovascular response to exercise over time.36 Another showed no decrease in 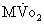 O2 with aging, but maximal oxygen consumption in these patients was significantly less than that of individuals without congenital heart disease.37 This finding was corroborated by a study using magnetic resonance imaging in a group of adult patients after atrial-level repair.38 Abnormal cardiac recovery after exercise was found despite the patients’ being in NYHA class I and receiving no medications. An echocardiographic study suggested that abnormalities in function during exercise might be due to impaired atrioventricular transport leading to reduced stroke volumes with stress.39
O2 with aging, but maximal oxygen consumption in these patients was significantly less than that of individuals without congenital heart disease.37 This finding was corroborated by a study using magnetic resonance imaging in a group of adult patients after atrial-level repair.38 Abnormal cardiac recovery after exercise was found despite the patients’ being in NYHA class I and receiving no medications. An echocardiographic study suggested that abnormalities in function during exercise might be due to impaired atrioventricular transport leading to reduced stroke volumes with stress.39
Late arrhythmias are common after the atrial switch procedures. At a median follow-up of 23 years, only two thirds of patients are in sinus rhythm, and two thirds have experienced supraventricular arrhythmias.32 Risk factors for supraventricular arrhythmias include pulmonary hypertension and a history of junctional rhythm. A separate study found arrhythmias in 22% of patients an average of 23 years after the Mustard procedure and that ventricular function was compromised in these patients compared with Mustard patients without arrhythmias, suggesting that late arrhythmias are a marker for systemic ventricular dysfunction.40 By 14 years after surgery, pacemakers were required in 22% of patients for either sick sinus syndrome or bradycardia because of heart block.41
Treatment of right ventricular dysfunction in these patients is evolving. Because of the hypertrophy of the right ventricle, some have suggested angiotensin-converting enzyme inhibition, which has been shown to be beneficial in left ventricular remodeling. Although there are some encouraging data for this population of patients, results have not been consistent.42 There are several surgical options to deal with late right ventricular failure after atrial switch: tricuspid valve repair or replacement if the ventricular dysfunction is associated with severe tricuspid regurgitation, conversion to an arterial switch, or heart transplantation. As severe right ventricular dysfunction is frequently if not always associated with severe tricuspid regurgitation in these patients, the first option seems an attractive one. The results, however, have been disappointing, with no significant improvement in function after surgery.29 Discussion of conversion to an arterial switch follows.
Arterial Repairs
The success with the atrial switch operations for TGA with intact septum did not translate to repair of transposition with large VSD. Disappointing results with VSD closure and atrial repair in this group of patients continued to be a stimulus for development of an arterial switch procedure that was first successfully performed by Jatene and colleagues in 1975 and subsequently reported by Yacoub.43,44 The success of the arterial switch procedure with reimplantation of the coronary arteries in some patients with TGA and VSD led to reintroduction of this technique for patients with intact ventricular septum. Yacoub’s initial attempts in patients with TGA and an intact ventricular septum were unsuccessful in 1972; however, additional reports by 1976 suggested that such repair was possible in infancy.45 Early mortality with the arterial switch in infancy was related to the development of pulmonary vascular obstructive disease and the complexity of the operation and to the fact that the left (pulmonary) ventricle was not prepared to sustain systemic pressure. Therefore, initial approaches included pulmonary arterial banding as a first stage, followed by arterial switch at a later time.45 However, improvements in the techniques of coronary transfer, myocardial protection, and neonatal vessel reconstruction have resulted in improved survival statistics in the arterial switch. Brawn, Castañeda, Quaegebeur, Yacoub, and their associates subsequently demonstrated the feasibility of repair of simple transposition in the first few days of life by arterial switch operation while the pulmonary ventricle has a relatively high pressure, producing results that rival or exceed those of atrial baffle operations.10,46–48
Technique
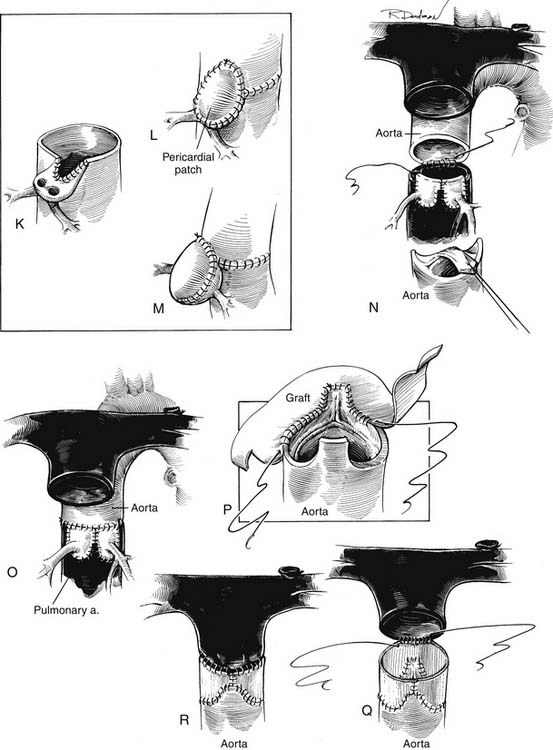
The principles of successful anatomic correction include dividing the aorta and pulmonary artery, excising the origins of the coronary arteries with a button of aortic wall, repositioning the coronary arteries to the posterior great vessel (pulmonary artery), and reconstructing each ventricular outflow to the appropriate distal vessel (Fig. 125-5). The details of the operative techniques, however, vary from one institution to another. The procedures are performed with a median sternotomy incision and the use of cardiopulmonary bypass and hypothermia, although hypothermic circulatory arrest is used for the procedure in varying degrees in many institutions. After the median sternotomy, a portion of the anterior pericardium is excised for autologous patch reconstruction of the defects in the anterior great vessel. The pericardium may be used fresh or fixed in glutaraldehyde to make it easier to manipulate during the operation. The ligamentum arteriosum or ductus arteriosus is dissected, and the pulmonary arteries are mobilized to the bifurcation of the vessels in the hilum of the lung bilaterally. It is important to mobilize the pulmonary arteries freely to permit anterior relocation of the pulmonary bifurcation without distortion of the pulmonary artery or aorta. The aorta is typically cannulated for bypass distally to allow room for manipulation of the proximal aorta during the reconstruction. After bypass is established, the ductus arteriosus is ligated and divided and the aortic end and pulmonary arterial end are oversewn as necessary. The aorta is then clamped close to the aortic cannula, and cardioplegia is administered into the aortic root.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree