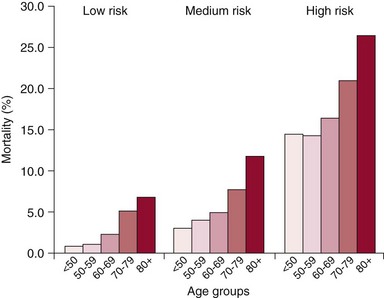
Chapter 22 Mitral regurgitation (MR) is a diverse disease that results from dysfunction of any of the portions of the complex mitral valve apparatus, including the leaflets, chords, annulus, and left ventricle. It is convenient to classify MR on the basis of two broad categories of dysfunction, namely primary (organic or degenerative) disease, which primarily affects the leaflets (e.g., fibromuscular dysplasia, mitral valve prolapse, rheumatic disease), and secondary (ischemic or functional) diseases, which spare the leaflets (e.g., diseases of the atrium and ventricle, including ischemic dysfunction and dilated cardiomyopathy) (see Chapters 18 and 19). More recently, it has been appreciated that even in secondary functional or ischemic MR, there may be changes that affect the leaflets. 1 Finally, some diseases such as ischemic MR may affect more than one portion of the valve apparatus. For example, both leaflet tethering and annular dilation may be present and may contribute to MR. 2 Patients with severe MR have decreased survival, 3 whether symptomatic 4 or not, 5 and surgery is often recommended. However, some studies have demonstrated that asymptomatic patients with severe MR and preserved left ventricular (LV) function can be safely monitored with a “watchful waiting” approach until the development of symptoms, LV dysfunction, pulmonary hypertension, or atrial fibrillation without a morbidity penalty at the time of surgery. 6 For these reasons, current guidelines recommend surgery for symptomatic patients and asymptomatic patients with abnormal LV function. 7 Surgery may also be considered for asymptomatic patients with normal LV function when there is a high likelihood of successful repair. Surgery to repair or replace the mitral valve in patients with severe MR appears to improve survival in observational studies. 8 However, the risks of surgery, particularly in consideration of morbidity and patient preference, have stimulated attempts to develop less invasive solutions. 9 Surgery is associated with mortality rates of 1% to 5% and additional morbidity rates of 10% to 20%, the morbidity including stroke, reoperation, renal failure, and prolonged ventilation. 10 Furthermore, in one study of Medicare-age patients, more than 20% required rehospitalization in the first 30 days after surgery. 11 The risks of surgery are particularly high in patients who are elderly or have LV dysfunction.10,12 In one study of more than 30,000 patients undergoing mitral valve replacement, the mortality increased from 4.1% in those younger than 50 years to 17.0% in octogenarians ( Figure 22-1). Similarly, significant morbidity (stroke, prolonged ventilation, renal failure, reoperation, sternal infection) occurred in more than a third of octogenarians. Predictors of risk in addition to age in this study included hemodynamic instability, severe symptoms, renal failure, and prior coronary artery bypass grafting surgery (CABG). 12 FIGURE 22-1 Mortality by age for low-, medium-, and high-risk categories of patients undergoing mitral valve replacement. (From Mehta RH, Eagle KA, Coombs LP, et al: Influence of age on outcomes in patients undergoing mitral valve replacement. Ann Thorac Surg 2002;74:1459-1467.) In patients with LV dysfunction and secondary MR, whether ischemic or functional, survival with or without surgery is not as good as in patients with preserved LV function and primary MR etiology. 4 Whether the increased mortality is a consequence of the preexisting LV dysfunction and whether the MR contributes to the reduced survival remain a controversial issues. In vitro studies have demonstrated progressive adverse LV remodeling in sheep even after successful MR repair. 13 Other studies have not shown benefit with annuloplasty repair of MR in dilated cardiomyopathy 14 or at the time of revascularization with CABG. 15 In both ischemic and nonischemic functional MR, age and comorbidities are the most important predictors of survival. 16 Thus, the major reason for surgery in most patients with ischemic MR is to provide symptomatic improvement and in those with primary MR to forestall the development of LV dysfunction. For this reason, it is essential to also discuss the efficacy of surgery in terms of MR reduction. In relatively young patients (mean age 55 to 60 years) with primary MR, long-term freedom from repeat surgery is well documented.17,18 However, recurrent 3+ and 4+ MR may occur in up to 30% of patients within 15 years.17,18 Recurrent MR is even more frequent in patients with ischemic MR, providing a potential target for the development of transcatheter therapies. 19 In keeping with the earlier discussion of the complexity of the mitral valve apparatus, it is useful to consider the percutaneous approaches according to the major structural abnormality that they address. 20 Unlike the extensive toolbox available to the mitral surgeon, transcatheter approaches are much more limited and often able to address only a single major element of the dysfunctional valve that contributes to MR. The remainder of this chapter addresses these transcatheter approaches with an emphasis on devices that have been approved in some part of the world, those that have entered first-in-human or phase 1 clinical investigation, and those with published data (either clinical or preclinical). Some devices that have been evaluated in vivo without success or are no longer under development are discussed only as they relate to other current approaches. Table 22-1 lists the devices along with their manufacturers, state of development, and any available published reports. The major technology in this category, MitraClip (Abbott Vascular), was also the first transcatheter mitral valve repair technology to receive CE Mark approval ( Figure 22-2). This system has its roots in the Alfieri stich operation, in which the middle scallops of the posterior and anterior leaflets (P2 and A2, respectively) are sutured together to create a double-orifice mitral valve. This operation, though usually performed with adjunctive ring annuloplasty, has proved effective and durable in a wide variety of pathologies and even in selected patients without annuloplasty.21,22 FIGURE 22-2 The MitraClip leaflet coaptation system. The concept of a percutaneous replicate of an Alfieri stich was initially conceived by St. Goar and subsequently developed as MitraClip by Evalve, Inc. (which was later acquired by Abbott Vascular). 23 A series of trials with this device confirmed its feasibility (Endovascular Valve Edge-to-Edge Repair Study [EVEREST] I), and its safety and efficacy were compared with those of surgical repair in a randomized trial (EVEREST II), providing a wealth of data on this technology as described later.24,25 The procedure is performed with standard catheterization techniques utilizing a transseptal approach from the right femoral vein. 26 The clip delivery system is introduced through a 24F sheath into the left atrium, where it can be guided using a series of turning knobs under transesophageal (both two- and three-dimensional) echocardiography guidance through the mitral valve into the left ventricle. A properly aligned and oriented clip can be placed on the P2 and A2 segments of leaflets, grasping them from the ventricular side to create leaflet opposition. Once leaflet insertion is confirmed by echocardiography, the clip can be released. If a suboptimal grasp occurs, the leaflet can be released, allowing repositioning prior to a second grasp attempt. Additionally, a second or more clips can be placed as needed for optimal MR reduction ( Figure 22-3). 26 FIGURE 22-3 Echocardiograms after deployment of two MitraClip devices. In the 2 : 1 randomized EVEREST II trial, 184 patients were designated to receive MitraClip therapy and 95 to undergo surgical repair or replacement. These patients were almost a decade older (mean age 67 years) than in usual surgical series and had more comorbidities. Major adverse events at 30 days were significantly less frequent with MitraClip therapy (9.6% versus 57% with surgery; P < 0.0001), although much of the difference could be attributed to the greater need for blood transfusions with surgery 27 ( Figure 22-4). The freedom from the combined outcome of death, mitral valve surgery, and MR severity greater than 2+ at 12 months was higher with surgery (73%) than with MitraClip therapy (55%; P = 0.0007). Importantly, in patients with acute MitraClip therapy success, the result appears durable with a very low rate of later mitral valve surgery ( Figure 22-5). FIGURE 22-4 Primary safety and efficacy endpoints for EVEREST II. FIGURE 22-5 The freedom from mitral valve (MV) surgery or reoperation after MitraClip implantation for patients in the randomized EVEREST II. Subsequent analyses of this rich database have demonstrated persistent reductions in MR grade, improvement in New York Heart Association (NYHA) functional class, and reduction in LV dimensions 27 with MitraClip therapy. Other studies have demonstrated a lack of mitral stenosis, no effect of initial rhythm on results, and benefit in higher-risk subjects.28–30 In the EVEREST II High-Risk Study, 78 patients with an estimated surgical mortality rate of 12% or higher (mean 14%) were treated with MitraClip, with an actual 30-day mortality of 8%. Survival at 12 months was 76% and significantly better than that of a concurrently screened comparison group, the majority of whom (86%) were treated medically. Patients treated with MitraClip had improved MR grade at 12 months (78% ≤2+), LV dimensions, New York Heart Association functional class, and quality of life, and a reduced need for hospitalization. 30 Similar benefit was demonstrated in another series of extreme-risk patients. 31 In addition, a group of European investigators have demonstrated the feasibility of MitraClip therapy in a group of 51 severely symptomatic patients with secondary ischemic or functional MR that failed to respond to cardiac resynchronization therapy. 32 Other devices discussed in this category are NeoChord, Mitra-Spacer, and MitraFlex ( Figure 22-6). The NeoChord DS1000 system is a transapically inserted tool that can capture a flail leaflet segment and pierce it with a semidull needle to attach a standard polytetrafluoroethylene (PTFE) artificial chord, which is then anchored to the apical entry site with a pledgeted suture. A first-in-human case has been reported, 33 and the device is currently undergoing a phase 1 evaluation in Europe in the Transapical Artificial Chordae Tendineae (TACT) trial. FIGURE 22-6 Additional leaflet repair technology. Mitra-Spacer (Cardiosolutions, Inc.) is an occluder device that is anchored in the LV apex via transseptal or transapical insertion with an anchor fixed outside the heart. The tethered “balloon-like” spacer floats in the mitral inflow pathway, providing a space occluder around which the mitral leaflets coalesce. This device has entered outside US first-in-human evaluation and has been deployed in four patients, with a reported reduction of one to two MR grades. 34 The MitraFlex device (Transcardiac Therapeutics) is designed as a transapically inserted thorascopic device to implant artificial chordae tendineae and is in preclinical development.
Transcatheter Mitral Valve Repair and Replacement
Rationale for Transcatheter Therapy
Classification of Percutaneous Repair Therapies
Leaflet and Chordal Technology
Mitraclip
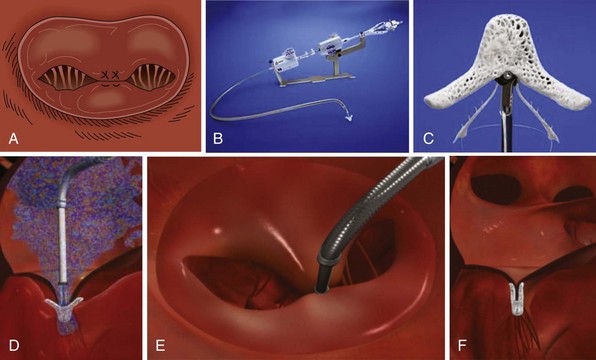
This device (Abbott Vascular, Inc.) creates a bridge between the P2 and A2 segments of the mitral valve similar to the Alfieri stitch operation (A) utilizing a clip delivery system (B) and the MitraClip (C). D and E, Side view and left atrial view of the clip delivery system as it is advanced through the mitral valve in the open position prior to grasping of the leaflets. F, The final result is illustrated after the clip has been released and the delivery system removed.
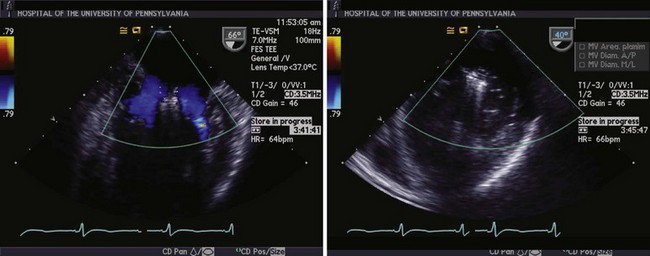
Left panel, Mitral inflow view demonstrating flow around the MitraClips (Abbott Vascular) into the ventricle through both orifices. Right panel, Dual orifices in the transgastric view.
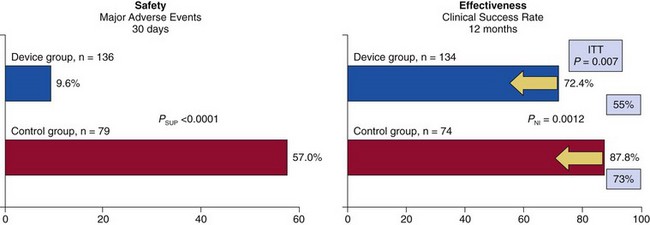
Rates of major adverse events at 30 days were reduced by MitraClip (Abbott Vascular) from 57.0% to 9.6% (P <0.0001). The rates of clinical success at 12 months for patients with immediate procedural success were similar, although by intent-to-treat (ITT) analysis of all patients (yellow arrows), effectiveness was better with surgery (73%) than with MitraClip (55%; p = 0.007). EVEREST, Endovascular Valve Edge-to-Edge Repair Study; NI, non-inferiority; SUP, superiority. (From Feldman T, Foster E, Glower D, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395-1406.)
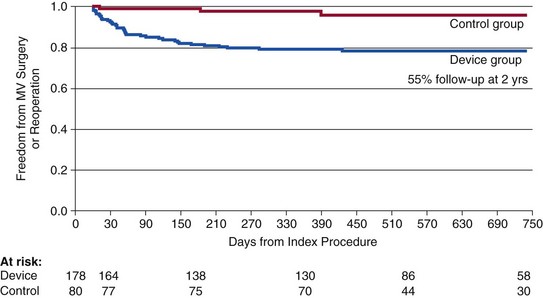
Note that patients with good results at 90 days after MitraClip (Abbott Vascular) appear to have a durable outcome to 2 years. EVEREST, Endovascular Valve Edge-to-Edge Repair Study. (From Feldman T, Foster E, Glower D, et al: Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011;364:1395-1406.)
Other Devices
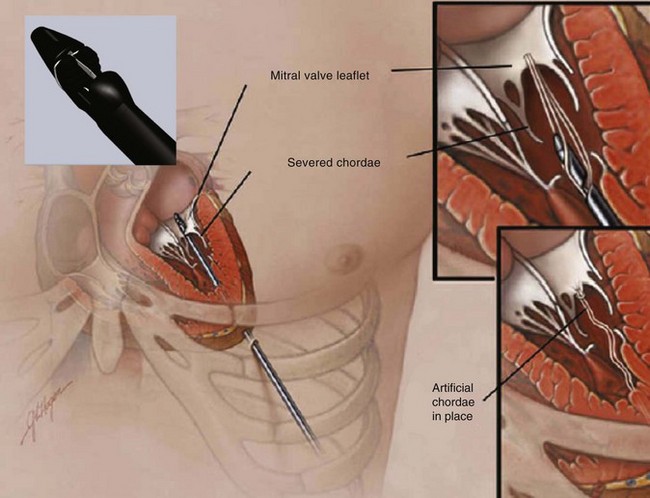
Neochord insertion of a transapically-anchored PTFE chord (NeoChord, Inc., Eden Praire, MN).
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Transcatheter Mitral Valve Repair and Replacement
Only gold members can continue reading. Log In or Register to continue