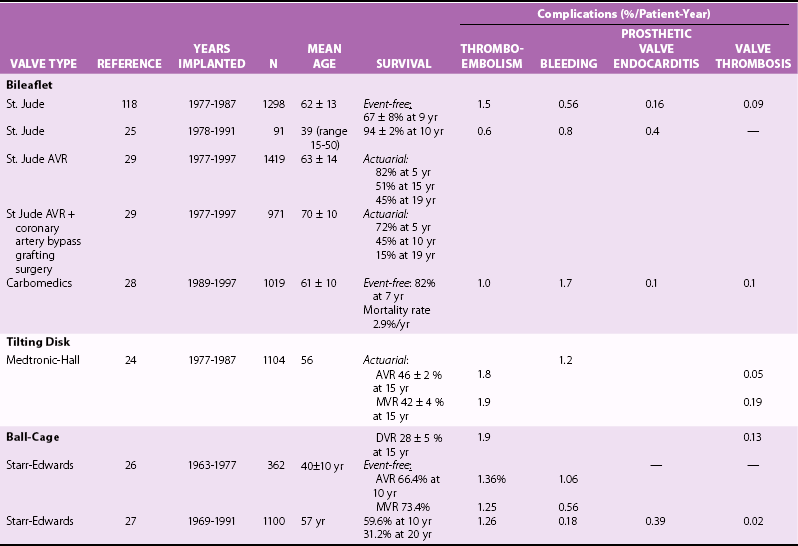
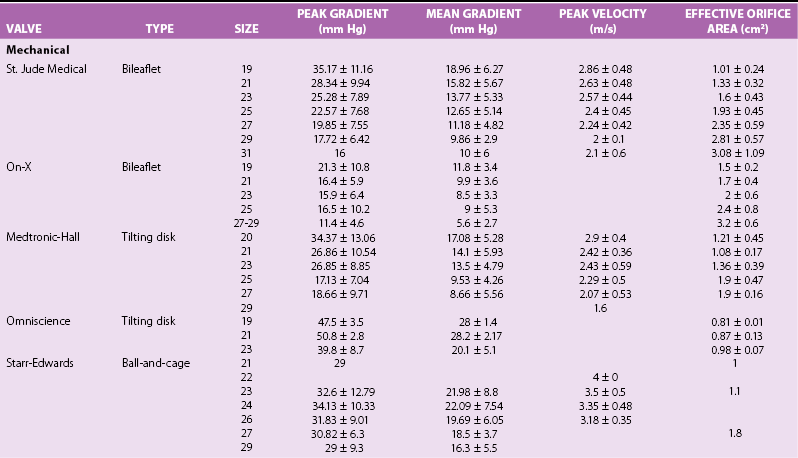
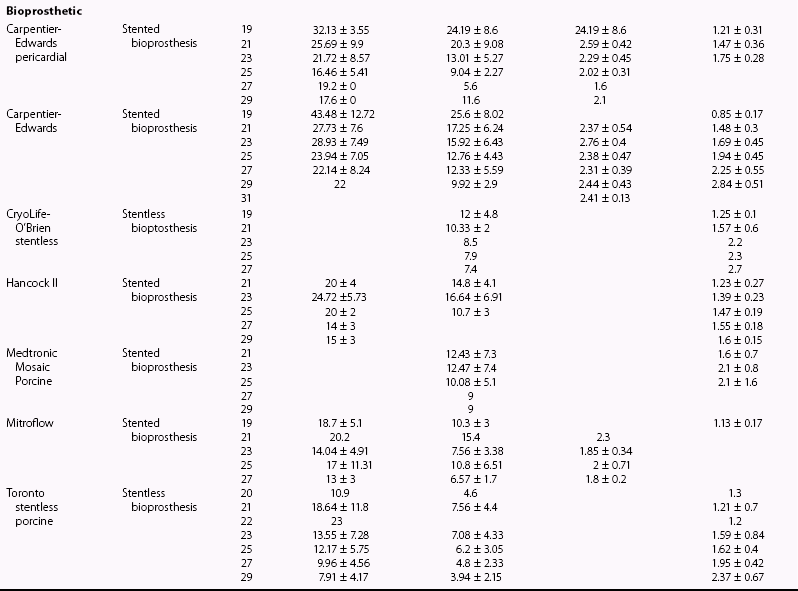
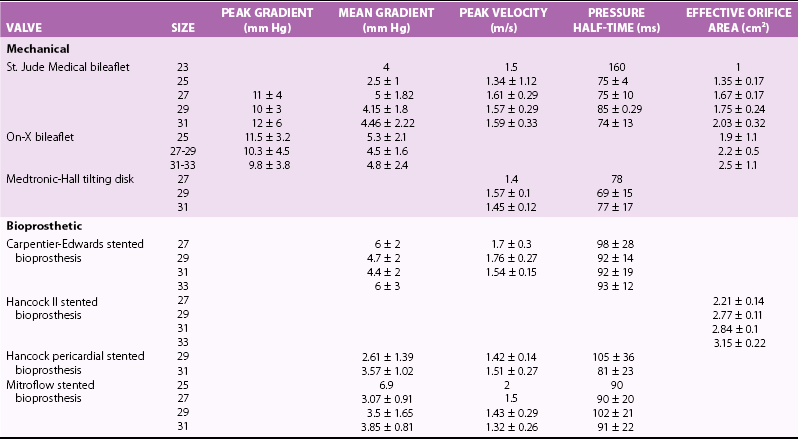
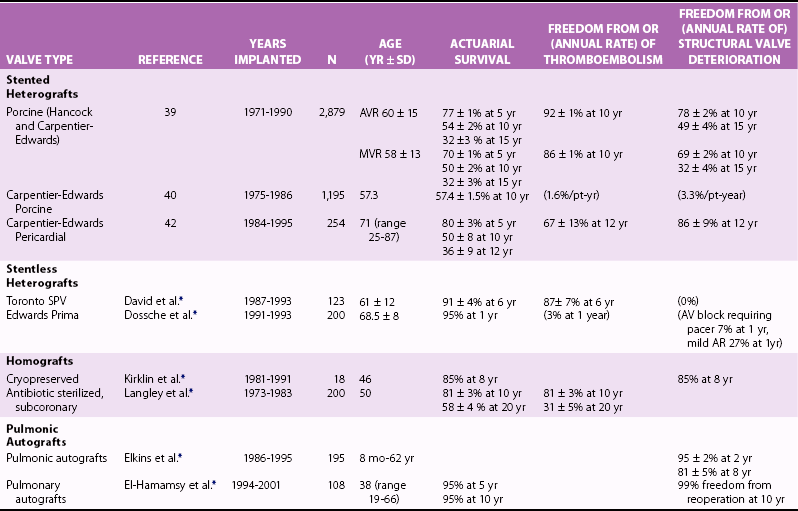
Chapter 26 The past six decades have witnessed extraordinary advancements in patient survival and functional outcomes following heart valve replacement surgery. Continued refinements in prosthetic valve design and performance, operative techniques, myocardial preservation, systemic perfusion, cerebral protection, and anesthetic management have enabled the application of surgery to an increasingly wider spectrum of patients. Minimally invasive surgical approaches and the aggressive use of primary valve repair when anatomically appropriate are now the routine in the vast majority of experienced centers. Heart valve teams have been formed to provide multidisciplinary assessment and treatment of patients with complex problems, including the use of transcatheter aortic valve replacement when appropriate. 1 Transcatheter mitral valve techniques remain under active investigation. More than 55,000 aortic or mitral valve replacement operations (with or without coronary artery bypass) were reported to the Society of Thoracic Surgeons’ National Adult Cardiac Surgery Database in calendar year 2011. 2 More than 25% of subjects with valvular heart disease in the 2003 Euro Heart Survey had undergone previous heart valve surgery. 3 Familiarity with the specific hemodynamic attributes, durability, thrombogenicity, and inherent limitations of currently available heart valve substitutes, as well as their potential for long-term complications, is critical to appropriate clinical decision making for patients in whom repair is not appropriate or feasible. The choice of valve prosthesis is inherently a trade-off between durability and risk of thromboembolism, with the associated hazards and lifestyle limitations of anticoagulation. The ideal heart valve substitute remains an elusive goal.4–6 Most surgical centers use a specific type of mechanical or tissue valve for the vast majority of their patients. Although standards have been developed for reporting outcomes after valve surgery, 7 comparisons of prosthetic valve performance are also heavily influenced by patient-, surgeon-, and institutional-related factors. 8 There are three basic types of mechanical prosthetic valves: bileaflet, tilting disk, and ball-cage ( Figure 26-1). The St. Jude bileaflet valve (St. Jude Medical, Inc., St. Paul, Minnesota) was first used in 1977 and is the most frequently implanted mechanical prosthesis worldwide. It consists of two pyrolytic semicircular “leaflets” or disks attached by hinges to a rigid valve ring. The open valve has three orifices: a small, tunnel- like central opening and two larger semi-circular orifies laterally. Its hemodynamic characteristics compare favorably to those of a tilting disk valve ( Tables 26-1 and 26-2). Performance indices (the ratio of effective orifice area to the area of the sewing ring) range from 0.40 to 0.70, depending on valve size. Effective orifice areas range from 0.7 cm2 for a 19-mm valve to 4.2 cm2 for a 31-mm prosthesis. Average peak velocities are 3.0 ± 0.8 meters per second (m/s) in the aortic position and 1.6 ± 0.3 m/s in the mitral position.9,10 Peak instantaneous gradients can be estimated using the modified Bernoulli equation, but mean gradient calculations are the more useful clinical parameter. The phenomenon of pressure recovery across bileaflet and ball-cage aortic valves magnifies the estimate of the difference between left ventricular (LV) and aortic pressures (i.e., the systolic gradient), especially when the latter is derived from measurements obtained close to the valve rather than more distally in the ascending aorta11–13 ( Figure 26-2). Additional confounding of the precise measurement occurs from the contribution of flow acceleration through the narrow central orifice of a bileaflet valve. 14 Thus, Doppler velocity determinations can overestimate the transvalvular gradient across bileaflet valves. Published reference tables of expected velocities for the various valve sizes should be consulted, and comparison with baseline postoperative studies made, to avoid misdiagnosis of prosthetic valve stenosis. 15 The Carbomedics valve (Sorin Group, Milan) is a variation of the St. Jude prosthesis that can be rotated to prevent limitation of leaflet excursion by subvalvular tissue. Both types of bileaflet valves have a small amount of normal regurgitation (“washing jet”) designed in part to decrease the risk of thrombosis. A small central jet and two converging jets emanating from the hinge points of the disks can be visualized on color-flow Doppler imaging.16–18 TABLE 26-1 Normal Doppler Echocardiographic Values for Selected Aortic Valve Prostheses Adapted from Rosenhek R, Binder T, Maurer G, et al. Normal values for Doppler echocardiographic assessment of heart valve prostheses. J Am Soc Echocardiogr 2003;16:1116–27. TABLE 26-2 Normal Doppler Echocardiographic Values for Selected Mitral Valve Prostheses Adapted from Rosenhek R, Binder T, Maurer G, et al. Normal Valves for Doppler echocardiographic assessment of heart valve prostheses. J Am Soc Echocardiogr 2003;16:1116–27. FIGURE 26-1 Mechanical heart valves. FIGURE 26-2 Pressure recovery. There are two principal tilting disk valves in clinical use. The Medtronic-Hall valve (Medtronic, Inc., Minneapolis, Minnesota) has a thin, circular disk of tungsten-impregnated graphite with pyrolytic coating, secured at its center by a curved, central guide strut, within titanium housing. The sewing ring is made of polytetrafluoroethylene (Teflon). The disk opens to 75 degrees in the aortic model and to 70 degrees in the mitral model. The Omniscience valve (Medical CV, Inc., Inner Grove Heights, Minnesota) disk is made of pyrolytic carbon and has a seamless polyester knit sewing ring. The disk opens to 80 degrees and closes at an angle of 12 degrees to the annular plane. For both valve types, the major orifice is semicircular in cross-section. Because the disk does not open to 90 degrees, there is slight resistance to flow with estimated pressure gradients of 5 to 25 mm Hg in the aortic position and 5 to 10 mm Hg in the mitral position 19 (see Tables 26-1 and 26-2). Effective orifice areas depend on valve size and range from 1.6 to 3.7 cm2, with performance indices of 0.40 to 0.65, similar to those reported for bileaflet mechanical valves. 20 Tilting disk valves also have a small amount of regurgitation, which arises from small gaps at the perimeter of the valve.16,21 With Medtronic-Hall valves, there is also a small amount of regurgitation around the central guide strut. 19 The bulky Starr-Edwards ball-cage valve, the oldest commercially available prosthetic heart valve (first used in 1965), is now very rarely implanted. Because of its sheer size, it is not suitable for use in the mitral position in patients with small LV cavities, in the aortic position in patients with small aortic root sizes, or for composite aortic valve-root reconstruction. The poppet is made of silicone rubber, the cage of Stellite alloy, and the sewing ring of Teflon/polypropylene cloth. The aortic cage is formed by three arches located at 120-degree intervals around the sewing ring. The ball-cage valve is more thrombogenic, and has less favorable hemodynamic performance characteristics, than both bileaflet and tilting disk valves. Antegrade flow occurs around the ball and through the struts of the cage. There is a small amount of regurgitant backflow before the ball seats following ejection. 19 Currently available mechanical valves have excellent long-term durability, with up to 45 years for the Starr-Edwards valve and more than 30 years for the St. Jude valve. In a randomized trial comparing the Starr-Edwards valve with the mechanical St. Jude valve, there were no differences in total or event-free survival rates through 8 years of follow-up for patients receiving either aortic valve replacement (AVR) or mitral valve replacement (MVR). 22 Structural deterioration, exemplified by some older-generation Bjork-Shiley (strut fracture with disk embolization) and Starr-Edwards (ball variance) prostheses, is now extremely rare. Ten-year freedom from valve-related death exceeds 90% for both St. Jude and Carbomedics bileaflet valves. 23 The Medtronic-Hall prosthesis has achieved comparable longevity. 24 Actuarial survival rates—which also depend importantly on several patient factors, such as age, gender, ventricular function, coronary artery disease, functional status, and major comorbidities—range from 94% ± 2% at 10 years for St. Jude valves, to 85 ± 3% at 9 years for Omniscience valves, and 60% to 70% at 10 years for Starr-Edwards valves25–31 ( Table 26-3). Long-term issues associated with mechanical valves include infective endocarditis, paravalvular leaks, hemolytic anemia, thromboembolism/valve thrombosis, pannus ingrowth, and hemorrhagic complications related to anticoagulation. All patients with mechanical valves require lifelong anticoagulation with a vitamin K antagonist (VKA), the intensity of which varies as a function of prosthesis type, position, and number. Bileaflet and current-generation tilting disk valves are significantly less thrombogenic than the ball-cage valve or older generation Bjork-Shiley valves. Higher-intensity anticoagulation is required for mechanical valves placed in the mitral versus the aortic position, for patients with multiple mechanical prostheses, and often for patients with additional risk factors for thromboembolism, such as atrial fibrillation (AF). Even with appropriately targeted anticoagulation, reported rates of thromboembolism range from 0.6 to 3.3 per 100 patient-years for patients with bileaflet or tilting disk valves.26,27,30–32 Complications related to anticoagulation in this population occur at rates of 0.9 to 2.3 per 100 patient-years. 33 A thromboembolism rate of 1.4 per 100 patient-years has been reported for the Starr-Edwards 1260 model valve. 29 The stented heterograft valve is a trileaflet valve with a circular opening in systole ( Figure 26-3). Porcine valves (e.g., Carpentier-Edwards, Hancock) are constructed of glutaraldehyde-fixed porcine aortic leaflets mounted on semisynthetic rigid or flexible stents and the sewing ring. One of the three leaflets of the porcine aortic valve is muscular and is typically replaced during construction with a fibrous leaflet from a second valve.34,35 There have been several iterative design improvements over time, including glutaraldehyde fixation at low or zero pressure, reconfiguration of the sewing ring, and treatments to retard calcification and reduce leaflet stiffness. The newer bovine pericardial valves (Carpentier-Edwards PERIMOUNT; see Figure 26-3) offer better hemodynamic performance than earlier-generation porcine bioprostheses (see Tables 26-1 and 26-2). In the aortic position, the antegrade velocity varies as a function of valve size but approximates 2.4 m/s, with a mean gradient of 14 mm Hg, and indexed valve area of 1.04 cm2/m2. 9 The pericardial aortic valve has a larger effective orifice area at any given valve size between 19 and 29. The average peak gradient in the mitral position is 9 ± 3 mm Hg and effective orifice area 2.5 ± 0.6 cm2. 36 A small degree of regurgitation can be detected by color-flow Doppler imaging in 10% of normally functioning bioprostheses. In a prospective randomized trial of patients with aortic valve disease, the Carpentier-Edwards PERIMOUNT Magna bovine pericardial valve (Edwards Lifesciences Corporation) demonstrated better hemodynamic performance and greater LV mass regression over 5 postoperative years than the newer-generation Medtronic Mosaic porcine valve (Medtronic, Inc.). 37 The major drawback with earlier-generation stented porcine valves was their limited durability, typically beginning within 5 to 7 years after implantation but varying with position and age at implant, with tissue changes characterized by calcification, fibrosis, tears, and perforations ( Figure 26-4). 38 Structural valve deterioration (SVD) occurs earlier for mitral than for aortic bioprosthetic valves, perhaps because of exposure of the mitral prosthesis to relatively higher LV closing pressures ( Table 26-4). The process of SVD is accelerated in younger patients, in those with disordered calcium metabolism (end-stage renal disease), and, possibly, in pregnant women independent of younger age ( Figure 26-5). In several older series, the estimated rate of SVD of porcine valves was 3.3% per patient-year, with freedom from valve failure at 10 years of 78% ± 2% for aortic valves and 69% ± 2% per patient-year for mitral valves.39–41 The rate of valve failure accelerates further after 10 years, such that the actuarial freedom from porcine bioprosthetic SVD is 49% ± 4% at 15 years for aortic valves and 32% ± 4% for mitral valves. 36 By comparison, the rate of freedom from primary tissue failure with pericardial aortic valves is 86% at 12 years ( Figure 26-6; see Table 26-4). 42 TABLE 26-4 Long-Term Outcome after Tissue Valve Replacement: Selected Series AV, Atrioventricular; AVR, aortic valve replacement; MVR, mitral valve replacement. *Data from: David TE, Feindel CM, Bos J, et al. Aortic valve replacement with a stentless porcine aortic valve: a six-year experience. J Thorac Cardiovasc Surg 1994;108:1030-6 ; Yun KL, Sintek CF, Fletcher AD, et al. Aortic valve replacement with the freestyle stentless bioprosthesis: five-year experience. Circulation 1999;100 (19 Suppl ): II17-23 ; Dossche K, Vanermen H, Daenen W, et al. Hemodynamic performance of the PRIMA Edwards stentless aortic xenograft: early results of a multicenter clinical trial. Thorac Cardiovasc Surg 1996;44:11-4; Kirklin JK, Smith D, Novick W, et al. Long-term function of cryopreserved aortic homografts: a ten-year study. J Thorac Cardiovasc Surg 1993;106:154-65; Langley SM, McGuirk SP, Chaudhry MA, et al. Twenty-year follow-up of aortic valve replacement with antibiotic sterilized homografts in 200 patients. Semin Thorac Cardiovasc Surg 1999;11(4 Suppl 1):28-34 ; Elkins RC, Lane MM, McCue C. Pulmonary autograft reoperation: incidence and management. Ann Thorac Surg 1996;62:450-5 ; El-Hamamsy I, Eryigit Z, Stevens LM, et al. Long-term outcoms after autograft versus homograft aortic root replacement in adults with aortic valve disease: a randomised controlled trial. Lancet 2010;376:524-31 FIGURE 26-3 Bioprosthetic heart valves. FIGURE 26-4 Bioprosthetic structural valve deterioration. FIGURE 26-5 Freedom from structural valve deterioration (SVD). FIGURE 26-6 Freedom from structural valve deterioration (SVD). The rigid sewing ring and stent-based construction of certain bioprostheses allow for easier implantation and maintenance of the three-dimensional relationships of the leaflets. However, these features also contribute to impaired hemodynamic performance and accelerated SVD. Stentless porcine valves (Toronto SPV [St. Jude Medical, Inc.], Edwards Prima; see Figure 26-3) were developed in part to address these issues. Their use has been restricted to the aortic position. Implantation is technically more challenging, whether in a subcoronary position or as part of a mini-root procedure, and hence these valves are preferred by only a minority of surgeons. Early postoperative mean gradients can be lower than 15 mm Hg, with further improvement in valve performance over time as a result of aortic root remodeling, lower peak exercise transvalvular gradients, and more rapid reduction in LV mass.43–49 There is a low incidence of important aortic regurgitation (AR), although results will vary as a function of technical expertise and appropriate valve sizing at time of implant. David et al 50 reported freedom from SVD with the Toronto SPV at 12 years of 69% ± 4%, 52% ± 8% for patients younger than 65 years, and 85% ± 4% for patients 65 and older. 50 This group has limited the use of this stentless valve to older patients with small aortic annuli. They have also emphasized the marked mortality hazard for reoperation for valve failure within 1 year of implantation. 50 Aortic valve homografts are harvested from human cadavers within 24 hours of death as blocks of tissue comprising the ascending aorta, aortic valve, a portion of the interventricular septum, and the anterior mitral valve leaflet. They are treated with antibiotics and cryopreserved at −196° C. 51 They are now most commonly implanted in the form of a total root replacement with reimplantation of the coronary arteries and trimming of any excess tissue not required for primary valve replacement. Sizing is based on echocardiographic measurement of the dimensions of the aortic annulus and sinotubular junction. Homograft valves appear resistant to infection and are preferred by many surgeons for management of aortic valve and root endocarditis in the active phase. Neither immunosuppression nor routine anticoagulation is required. Despite earlier expectations, long-term durability of such homografts beyond 10 years is not superior to that for current-generation pericardial valves.52–54 In an echocardiographic follow-up study of 570 patients with aortic valve homografts, 72% had signs of valve dysfunction at 6.8 ± 4.1 years after implantation, with moderate to severe AR in 15.4 %, moderate aortic stenosis (AS) in 10%, and severe aortic stenosis in 2.5%. 55 Rates of homograft reoperation at 15 years for SVD, which do not account for all cases of SVD, approximate 20% for patients 41 to 60 years of age and 16% for those older than 60 years at time of implantation. 56 Excessive leaflet and root calcification renders reoperation particularly challenging. Mitral homograft valve replacement, a considerably complex technical feat, is not advocated. In the Ross procedure, the patient’s own pulmonic valve or autograft is harvested as a small tissue block containing the pulmonic valve, annulus, and proximal pulmonary artery, and is inserted in the aortic position, usually as a complete root replacement with reimplantation of the coronary arteries.57–66 The pulmonic valve and right ventricular outflow tract are then replaced with either an aortic or pulmonic homograft. Thus, the procedure requires two separate valve operations, a longer time with the patient on cardiopulmonary bypass, and a steep learning curve. With appropriate selection of young patients by expert surgeons at experienced centers of excellence, operative mortality rates are less than 1% and freedom from valve–related death is as high as 84% ± 6% at 14 years.67–70 Advantages of the autograft include the ability to increase in size during childhood growth, excellent hemodynamic performance characteristics, lack of thrombogenicity, and resistance to infection. The hemodynamic performance characteristics of the pulmonary autograft are similar to those of a normal, native aortic valve both at rest and with exercise. However, the homograft in the pulmonic position has a higher mean gradient at rest (9 ±7 mm Hg) and with exercise (21 ± 14 mm Hg) than a normal, native pulmonic valve.71–73 Early homograft stenosis occurs in 10% to 20% of patients and is due to extrinsic compression from inflammation and adventitial fibrosis.74,75 The procedure is usually reserved for children and young adults but should be avoided in patients with dilated roots, given the unacceptably high incidence of accelerated degeneration and pulmonary autograft dilation with significant regurgitation. The Ross procedure is not practiced widely, and surgical opinions differ regarding the best approach to the child or young adult with aortic valve disease. The durability of the operation has also been called into question; the rate of SVD at 10 years approximates 30%.54,61,67–69,76–79 Reoperation can be especially hazardous. 80 The use of pericardial autograft valves for either aortic or mitral replacement, in which the patient’s own pericardium is fashioned onto a frame in the operating room, has had very limited support despite excellent hemodynamic performance characteristics and durability in small patient subsets. 81 Obvious differences between valve types relate to durability (i.e., indefinite for mechanical versus limited for tissue valves) and need for anticoagulation (i.e., obligatory for mechanical versus none for tissue valves in the absence of other risk factors for thromboembolism). Short- to intermediate-term hemodynamic performance characteristics for low-profile mechanical prostheses (e.g., St. Jude) are comparable to those for stented tissue valves of similar size. There are no important differences in rates of prosthetic valve endocarditis (PVE), although some series have suggested a higher incidence of early (<1 year) infection with mechanical valves than with tissue heterografts. 82 Two earlier randomized trials compared long-term outcomes with a spherical tilting disk valve (Bjork Shiley) and with a stented porcine valve (Hancock or Carpentier-Edwards). In the Veterans Affairs trial, 575 men were randomly assigned to one of the two groups between 1977 and 1982; 394 underwent AVR, and 181 MVR. 83 Among those undergoing AVR, survival at 15 years was better with mechanical AVR (34% ± 3% vs. 21% ± 3%, P = 0.02; Figure 26-7), whereas there was no difference in survival between mechanical and tissue MVR procedures (81% and 79%, respectively). With AVR, the increased mortality among patients allocated a tissue prosthesis was driven largely by the higher rate of SVD and risks associated with reoperation. SVD occurred predominantly in patients younger than 65 years, beginning at 5 to 6 years after MVR and 7 to 8 years after AVR. In patients undergoing AVR, the cumulative incidence of SVD was 23% ± 5% for tissue valves and 0% ± 0% for mechanical valves, and in patients undergoing MVR, 44% ± 8% for tissue valves and 5% ± 4% for mechanical valves. There was an increased risk of bleeding with mechanical valve replacement, but no significant differences were observed for other valve-related complications, such as thromboembolism and PVE. In the Edinburgh Heart Valve Trial, 541 men and women were randomly allocated to treatments between 1975 and 1979 and followed up for a mean of 12 years after AVR (n = 211), MVR (n = 261), or AVR + MVR (n = 61). 84 There was a trend toward improved survival with mechanical valve replacement (P = 0.08) and higher rates of reoperation with tissue valve replacement (AVR 22.6 ± 5.7% vs. 4.2 ± 2.1%, P <0.01; MVR 43.1 ± 6.0% vs. 9.9 ± 3.2%, P <0.001). Bleeding rates were higher with mechanical AVR, but there were no differences in rates of thromboembolism or PVE. A meta-analysis found no differences in survival between mechanical and tissue valves when patient age and risk factors were included in the model. 85 A later, smaller randomized trial of 313 patients 55 to 70 years of age with aortic valve disease also found no difference in late survival between newer-generation mechanical and tissue prostheses, with higher rates of SVD and reoperation in patients with tissue valves but no other differences in secondary end points. 86 In an analysis of data from more than 39,000 patients aged 65 to 80 years undergoing AVR reported to the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database and linked to Medicare, patients receiving a bioprosthesis had similar adjusted risks for death, higher risks for reoperation and endocarditis, and lower risks for stroke and bleeding in comparison with patients receiving a mechanical valve. Of note, patients aged 65 to 69 years had a substantially elevated 12-year absolute risk of reoperation (10.5%). 87 In a separate analysis of the same database, the proportion of patients older than 70 years who received a tissue prosthesis at the time of valve replacement increased from 87% to 96% between 2004 and 2009 without any associated rise in rates of adverse events. 88 FIGURE 26-7 Veterans Affairs randomized trial results.
Prosthetic Heart Valves
Mechanical Valves
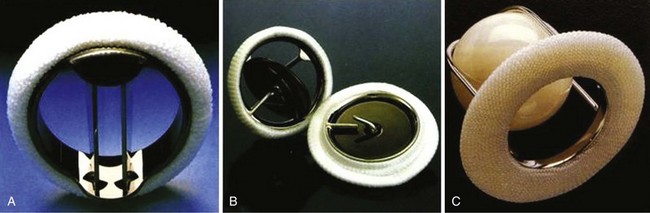
A, St. Jude bileaflet valve (St. Jude Medical, Inc., St. Paul, Minnesota). The occluding mechanism consists of two semicircular leaflets that pivot apart during systole, creating three separate orifices as shown. B, Medtronic-Hall tilting disk valve (Medtronic, Inc., Minneapolis, Minnesota). The disk opens to 75 degrees in the aortic model and 70 degrees in the mitral model. It is retained by an S-shaped center guide strut. C, Starr-Edwards ball-cage valve (Edwards Lifesciences Corporation, Irvine, California). The poppet is made of siliconized rubber. The sewing ring is more generous than those with bileaflet or tilting disk valves. (From Antunes MJ, Burke AP, Carabello B, et al. In: Rahimtoola SH, editor. Valvular heart disease. Philadelphia: Current Medicine; 2005. p. 296–7. Braunwald E, series editor. Essential atlas of heart diseases. 3rd ed. vol. XI.)
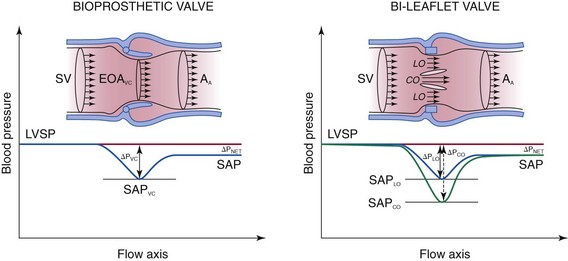
Velocity and pressure changes from the left ventricular (LV) outflow tract to the ascending aorta (AA) in the presence of a stented bioprosthesis (left) and a bileaflet mechanical valve (right). Because of pressure recovery, velocities are lower and systolic aortic pressure (SAP) is higher in the distal aorta than at the level of the vena contracta (VC). This phenomenon is more exaggerated in the example of the bileaflet mechanical valve because the velocity is higher in the central orifice (CO), where the pressure drop is higher. Doppler gradients are estimated from the maximal velocity at the level of the vena contracta and represent the maximal pressure drop, whereas catheterization measurements reflect the systolic pressure difference (ΔP) between the left ventricle (LV) and the ascending aorta. EOA, Effective orifice area; LO, lateral orifice; SP, systolic pressure; SV, stroke volume. (Adapted from Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocardiogr 2009;22:975–1014.)
Durability and Long-Term Outcomes
Tissue Valves
Stented Heterograft Valves
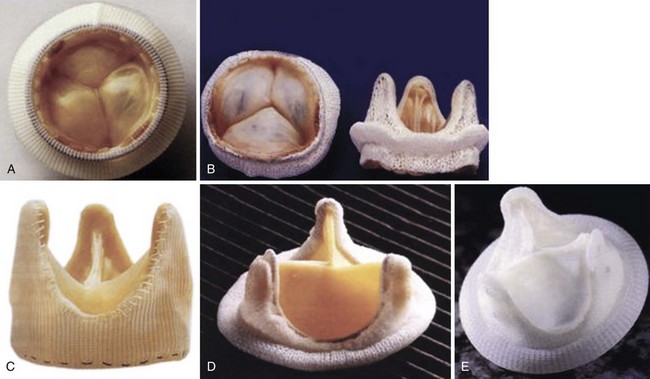
A, Hancock modified-orifice (MO) stented valve (Medtronic, Inc., Minneapolis, Minnesota). The MO valve is produced by replacing the muscular right coronary cusp with the noncoronary cusp from another porcine valve. B, Carpentier-Edwards stented porcine valve (Edwards Lifesciences Corporation, Irvine, California). The annulus is purposefully asymmetric to obliterate the muscular septal ridge of the porcine right coronary cusp. C, St. Jude Medical Toronto SPV stentless valve (St. Jude Medical, Inc., St. Paul, Minnesota). D, Carpentier-Edwards pericardial valve (Edwards Lifesciences Corporation). E, Autologous pericardial valve. (From Antunes MJ, Burke AP, Carabello B, et al. In: Rahimtoola SH, editor. Valvular heart disease. Philadelphia: Current Medicine; 2005. p. 296–7. Braunwald E, series editor. Essential atlas of heart diseases, 3rd ed. vol. XI.)
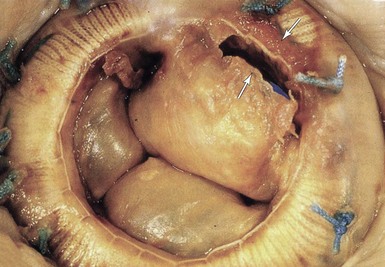
Five-year-old mitral Hancock porcine valve. There is a linear tear at the base of one of the 3 cusps (white arrows). (From Antunes MJ, Burke AP, Carabello B, et al. In: Rahimtoola SH, editor. Valvular heart disease. Philadelphia: Current Medicine; 2005. p. 296–7. Braunwald E, series editor. Essential atlas of heart diseases. 3rd ed. vol. XI.)
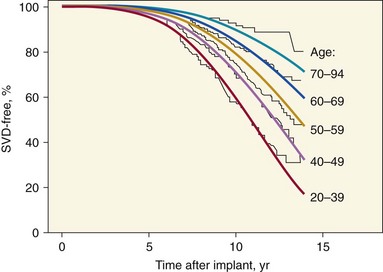
Actuarial freedom from SVD for 4910 operative survivors of isolated aortic or mitral valve replacement with Hancock (Medtronic, Inc., Minneapolis, Minnesota) or Carpentier-Edwards (Edwards Lifesciences Corporation, Irvine, California) porcine valves. The curves are stratified by age group and show a significantly lower rate of SVD for older than for younger patients. A Weibull regression model based on patient age and valve position (smooth lines) was used to fit the actuarial Kaplan-Meier curves (jagged lines). (Adapted from Grunkemeier GL, Jamieson WRE, Miller DC, et al. Actual vs. actuarial risk of structural valve deterioration. J Thorac Cardiovasc Surg 1994;108:709–18.)
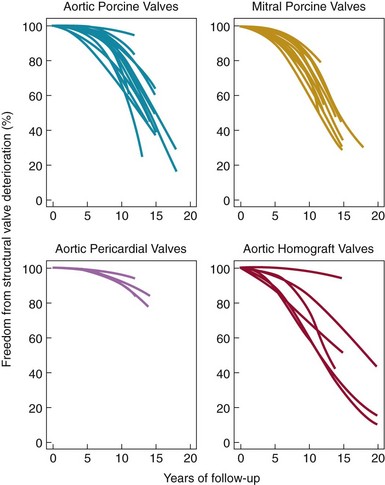
Weibull distribution curves for freedom from SVD for four types of tissue valves. Note the more gradual rate of SVD for aortic pericardial valves. (Adapted from Grunkemeier GL, Li H-H, Naftel DC, et al. Long-term performance of heart valve prostheses. Curr Prob Cardiol 2000;25:73–156.)
Stentless Heterograft Valves
Homografts
Autografts
Comparison of Mechanical and Tissue Valves
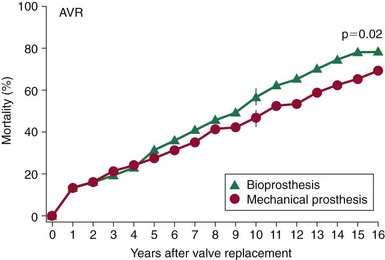
Mortality rates after aortic valve replacement with a mechanical prosthesis (Bjork-Shiley, Pfizer, Inc., New York) and a stented porcine prosthesis (Hancock, (Medtronic, Inc., Minneapolis, Minnesota)). At 15 years, mortality was 66 ± 3% for mechanical valve vs. 79 ± 3% for the porcine valve P = 0.02). (From Hammermeister K, Sethi GK, Henderson WG, et al. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veteran Affairs randomized trial. J Am Coll Cardiol 2000;36:1152–8.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree