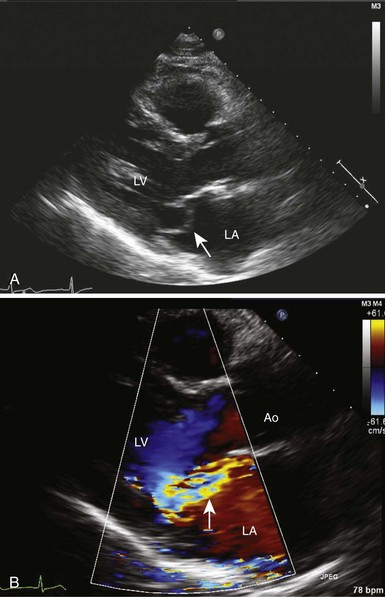
Chapter 18 ANATOMY OF THE NORMAL AND MYXOMATOUS MITRAL VALVE ETIOLOGY AND PATHOLOGY OF MITRAL VALVE PROLAPSE EPIDEMIOLOGY AND NATURAL HISTORY DIAGNOSIS AND CLINICAL FEATURES MITRAL VALVE PROLAPSE SYNDROME MANAGEMENT OF THE ASYMPTOMATIC PATIENT COMPLICATIONS OF MITRAL VALVE PROLAPSE Mitral valve prolapse is a common disorder that has been recognized as a specific condition since the 1960s, when Barlow and Bosman 1 used cineangiography to delineate the cause of systolic clicks and murmurs. Previously, myxomatous change in mitral valve tissue had been recognized pathologically. However, it was only with the arrival of two-dimensional (2D) echocardiography in the 1970s and subsequently that the natural history and pathophysiology of the condition and its complications became manifest. Mitral valve prolapse (MVP), a term originally coined by Criley et al, 2 is now recognized as the major cause of mitral regurgitation (MR) in developed countries and a cause of premature mortality and considerable morbidity if not diagnosed and appropriately managed.3,4 In this chapter, we outline the pathogenesis of myxomatous mitral valve disease, its natural history and clinical manifestations, and the current approach to diagnosis and management of the disease and its complications. One of the difficulties in diagnosing and managing patients with MVP is appropriate patient classification. Myxomatous mitral valve disease and MVP are conditions that occur together but are not necessarily synonymous ( Figure 18-1).5,6 Understanding the interplay between these entities is essential. Myxomatous mitral valve disease is a pathologic condition in which the mitral valve leaflets and chordae are thickened, there is hooding of the leaflets, and abnormal accumulations of mucopolysaccharides are seen in chordae and leaflets.3,7 The valve abnormalities and especially the chordal elongation produce prolapse of the leaflets recognized echocardiographically that, in some cases, leads to MR. 8 A systolic click and murmur are characteristic clinical findings. 9 This condition is associated with complications in a substantial minority of patients affected and may eventually lead to the need for valve surgery.10–12 However, myxomatous mitral valve disease may exist in a preclinical phase without any overt echocardiographic or clinical manifestations. Barlow disease defines the situation of significant and diffuse myxomatous degeneration leading to bileaflet redundancy and broad bileaflet prolapse. FIGURE 18-1 Interaction of myxomatous disease of the mitral valve, which is diagnosed on pathologic examination, and mitral valve prolapse, which is diagnosed on an echocardiogram. Observation of superior displacement of part of a mitral valve leaflet in systole on 2D echocardiography is common even in normal people. Before the refinement of echocardiographic criteria, prolapse was diagnosed in a substantial portion of the population. 13 Even with stricter echocardiographic criteria for diagnosis, superior displacement of the mitral valve leaflets is seen in the absence of leaflet thickening or MR in some normal people. This form of prolapse appears to arise in many instances from a disproportion between mitral leaflet size and left ventricular (LV) size. For example, it can be seen in patients with a small left ventricle and may subsequently disappear with volume loading.14–16 It can also be seen in patients with an atrial septal defect, and may disappear on closure of the septal defect.17,18 This subtle type of prolapse is not a precursor of myxomatous changes in the mitral valve and appears to have a very benign prognosis. A basic knowledge of the anatomy of the normal mitral valve is important to understanding and recognizing the variable presentation of myxomatous mitral valve disease.19,20 The mitral valve consists of anterior and posterior leaflets attached at their bases to a fibrous or fibromuscular ring, the mitral annulus ( Figure 18-2). The leaflets in turn are attached to the two papillary muscles (anterolateral and posteromedial) by chordae tendineae, one of whose functions is to prevent eversion or prolapse of the leaflets in systole. FIGURE 18-2 Normal mitral valve. The anterior leaflet is generally larger than the posterior leaflet and is triangular. The anterior area leaflet has two distinct portions, a thin translucent area at the base and a more opaque thicker area at the free edge (the rough zone) where coaptation with the posterior leaflet occurs. The posterior leaflet is smaller, has a longer attachment to the annulus, and is generally segmented by clefts at the free edge into three segments or scallops. The anterior and posterior leaflets meet at the two commissures (posteromedial and anterolateral) and are fused there by a rim of valve tissue of variable (<1 cm) width. Carpentier 21 has provided a surgical classification of mitral valve anatomy that is widely used. In this classification, the mitral valve has six segments, three in the anterior leaflet (A1, A2, and A3) and three in the posterior leaflet (P1, P2, and P3). The posterior leaflet segments consist of the naturally occurring scallops (P1, lateral; P2, middle; and P3, medial); the anterior segments constitute the areas adjoining each of the three posterior scallops ( Figure 18-3).21,22 FIGURE 18-3 Mitral valve as viewed from the left ventricle. Normal chordae vary widely in number and appearance, and each papillary muscle provides chordae to both leaflets. Primary chordae are attached to the valve at the leaflet edge and serve to halt valve-edge prolapse. Secondary chordae attach the papillary muscle to the ventricular surface of the leaflets at the region of coaptation. Their role is to anchor the leaflets, and they are integral to optimal ventricular function (highlighting the idea of valvuloventricular synergy and providing a rationale for valve-sparing mitral valve replacement techniques). Of note, the secondary chords that attach to the anterior leaflet are more prominent than those attaching to the posterior leaflet. The tertiary chords arise directly from the ventricular trabeculae and attach to the posterior leaflet annulus. Additional chordae are attached at the commissures and at each cleft of the posterior mitral leaflet. The chordae to the posterior leaflet are inserted into each of the scallops, explaining why chordal rupture may lead to prolapse or flail of an individual scallop. On average, 25 major chordal trunks attach the leaflets to the papillary muscles, equally divided between anterior and posterior; an additional 100 smaller chords also attach to the leaflets. 23 Myxomatous disease of the mitral valve may affect one or both leaflets and may affect many or only some of the chordae. Prolapse or flail of the posterior leaflet is the most common indication for surgical intervention. In one study of more than 1000 patients undergoing surgery for myxomatous MR, more than 50% had evidence of chordal rupture to the posterior leaflet. 24 The middle scallop (P2) is the segment most commonly affected, followed by the lateral and then the medial scallop. MVP is a degenerative condition of the mitral valve that usually becomes evident in adulthood and is associated with a number of connective tissue disorders. Each of these disorders is related to mutations in extracellular matrix genes, thus suggesting a role for abnormalities of structural proteins in the pathogenesis of MVP.19,25–29 MVP is seen in patients suffering with the Ehlers-Danlos syndrome and osteogenesis imperfecta, both of which are associated with mutations in collagen. Patients with the Marfan syndrome have a mutation in the fbn1 gene encoding fibrillin, a major component of elastin, and also display MVP. In a related pathway, mutations in transforming growth factor-β (TGF-β) signaling may be responsible for the progression of Marfan syndrome and also result in the Loeys-Dietz syndrome, which is associated with a number of phenotypes, including MVP. Whether abrogation of the TGF-β pathway using angiotensin receptor blockade, as shown in Loeys-Dietz mice with aortic pathology, can slow the progression of MVP in humans is unknown but is an interesting concept. Ultimately, however, the majority of MVP cases are idiopathic in nature, and although characteristic abnormalities on both gross pathologic and histologic examinations are evident, the precise mechanism of disease remains to be determined. Pathologically, myxoid degeneration of valve tissue is distinctive. 30 Grossly, there is enlargement and thickening of the leaflets and chords, interchordal hooding of the leaflets, and annular dilation with elongated and frequently ruptured chordae ( Figure 18-4). The tissue has a spongy texture, and valve thickening is in large part due to the deposition of proteoglycans and collagen. The redundancy of leaflet tissue is often substantial, and fibrin deposits and even microthrombi may be evident in the folds at the base of the leaflets.31–33 In addition to these microthrombi, platelet activation at the site of roughened endothelium in the valve has also been postulated as a source of emboli. 32 The increased motion of valves and chords often leads to fibrosis of both valve tissue and endocardium at points of contact in the atrium and ventricles. These fibrosed areas have been postulated by some to be a source of increased arrhythmogenicity. 34 FIGURE 18-4 Gross appearance of myxomatous valve and chordae illustrating the thickening and spongy appearance of the leaflets and chordae. The mitral valve has three layers histologically: the atrialis, a layer of collagen and elastic tissue that forms the atrial aspect of the leaflet; the spongiosa, a middle layer that contains structural proteins and proteoglycans; and the fibrosa, or ventricularis, that consists predominantly of collagen and is on the ventricular side of the leaflet ( Figure 18-5). 35 In myxomatous disease, the spongiosa shows an accumulation of proteoglycans and glycosaminoglycans that extends into the chords and the fibrosa and has reduced collagen staining. 36 The increased extracellular matrix gives the tissue a blue color on hematoxylin and eosin staining and was the original basis for the “myxomatous” label. The increased proteoglycans in the fibrosa are postulated to interfere with tensile strength. An inflammatory infiltrate is not seen in myxomatous tissue, but myofibroblasts are modulated to a more activated format. 37 FIGURE 18-5 Histologic appearance of a mitral valve leaflet. The myxomatous mitral valve has been classified into two types on the basis of its surgical appearance by Carpentier’s group. 38 One type, called Barlow disease, is seen in younger patients with marked redundancy of the tissue and prolapse that may involve multiple segments and is more difficult to repair.31,39 This type is also seen in connective tissue disorders such as Marfan syndrome. Fibroelastic deficiency, the other type, is seen in older patients in whom myxomatous changes are confined to a single segment, typically the posterior middle scallop (P2). The rest of the valve does not appear myxomatous. There is considerable overlap between these groups, and in our and others’ experience it has proved difficult to differentiate the groups reproducibly on either the gross or histologic appearance of the valve. 38 At a mechanical level, considerable abnormality is noted in myxomatous valve disease, which in part explains the pathophysiology. Fragmentation and irregularity of both collagen and elastin have been reported.40–42 When myxomatous valve tissue obtained at the time of mitral valve surgery is subjected to formal stress/strain analysis, the leaflets are more extensible and less stiff than normal but have relatively minor reductions in tensile strength. 43 Tensile strength is seriously compromised in the chordae despite the increase in thickness and extensibility. Myxomatous chordae fail at 25% of the load that it takes to rupture a normal chord. 44 They exhibit areas of thickening due to glycosaminoglycans deposition and fibrous sheath formation. 45 The poor load-bearing qualities of these chordae suggest that the collagen in the fibrous sheath does not add any tensile strength. 46 Biochemically, alterations in myxomatous tissue have also been noted. The major abnormalities relate not only to the increased amounts of glycosaminoglycans but also to the types produced. Glycosaminoglycans have multiple roles, including imparting specific qualities to connective tissue and modifying the structure of collagen. Glycosaminoglycans are incorporated with proteins as proteoglycans, which have also inherent specific properties. 47 These properties may in turn be further modified by combination with other proteoglycans. In myxomatous chordae, the amount of glycosaminoglycans is twice the normal amount, without any change in cellularity, suggesting that this increase occurs because of either greater production or reduced degradation or a combination. Thus far, mutations in proteoglycans have not been identified in association with MVP, but it is possible that improper processing of these proteins leads to degenerative valve disease (as shown in Adamts9 protease-deficient mice). 48 As in the mechanical findings, the degree of biochemical abnormality in myxomatous tissue is more marked in chordal tissue than in the leaflets. 47 Although the individual glycosaminoglycans in valve and chordal tissue are similar, their proportions differ substantially. These proportions are further altered in myxomatous tissue, as is the chain length of the glycosaminoglycans themselves. In myxomatous chordae, there is an excess of hyaluronan and chondroitin-6-sulfate. Hyaluronan and chondroitin-6-sulfate are constituents of the proteoglycan versican. Versican is a large proteoglycan that in combination with hyaluronan is thought to increase hydration and sponginess of connective tissue. These properties are ideal to withstand compressive forces such as those that occur at the coaptation surface. In addition, myxomatous chordae have decreased amounts of 4-sulfated glycosaminoglycans commonly seen in the small proteoglycan decorin. Decorin is important in collagen fibrillogenesis, and its reduction may further explain the decrease in the tensile strength encountered in these chordae. 49 Interestingly, the proportions of glycosaminoglycans seen in myxomatous chordae more closely resemble those in normal leaflets than those in normal chordae. It is unclear why this finding occurs, whether it is genetically determined or whether local environmental factors in the valve itself play a role. The finding that glycosaminoglycan production is modulated in a cell culture model of valve tissue by alterations in environmental factors such as tissue strain and location suggests that glycosaminoglycan synthesis or degradation is responsive to local environmental conditions. 50 A neural network that is impaired or damaged in the myxomatous process has also been described in valves. 51 This impairment too, could potentially be involved in aligning appropriate glycosaminoglycan production with a sensed physiologic stimulus such as tension or compression. In addition to changes in glycosaminoglycans, an increase in matrix metalloproteinases and other degradative enzymes is reported in myxomatous mitral valve disease. 37 These enzymes appear to be produced by cells within the valve tissue, are capable of structural protein degradation, and may play a role in the structural abnormalities in this disease or may occur in response to the alterations caused by the disease. Myxoid degeneration of the mitral valve is often familial, although the severity of expression varies considerably within a given family, suggesting that environmental influences are also important.52,53 The inheritance is considered to be autosomal dominant with variable penetrance that is influenced by both age and gender. It is uncommon in our experience at Cleveland Clinic to perform surgery for myxoid MR on more than one member of an affected family despite the familial nature of the disease. Work from Devereux’s group suggested that although MVP occurs with frequency in the families of those affected, the severity of the lesion is very variable within the same kindred.53,54 In one study, probands with thickened leaflets were more likely to have family members with MVP (53%) than those without significant leaflet thickening (27%). In multiple studies, heterogeneity of findings in relatives of probands is seen. Thus, leaflet thickening or involvement of one or both leaflets may vary in family members, suggesting that phenotypic expression is affected by many other factors rather than a specific genotype. 53 At least three separate loci for the MVP trait have been identified in extended families with multiple affected members. These include a locus identified in 1999 in France (MMVP1), which was mapped to chromosome 16p11.2-p12.1. 55 A further locus, MMVP2, was identified in 2003 by Freed et al 56 on chromosome 11p15.4. A third locus (MMVP3) on chromosome 13 (13q31.3-q32.1) was reported by Nesta et al 57 in a family of 43 members, of whom 9 had conventional diagnostic criteria for MVP. Prolapse configuration was not uniform in those affected, and both thickened and nonthickened prolapsing leaflets were seen; prolapse involved the posterior leaflet in some and both leaflets in others. The proteins coded by the three reported MVP loci are unknown as yet because these studies were performed with linkage analysis. 58 However, the locus on chromosome 13 has genes of potential interest that involve cell growth and differentiation. The occurrence of myxomatous MVP in association with other inherited connective tissue disorders raised the question whether proteins involved in these disorders might underlie idiopathic myxomatous degeneration. So far, however, the genes encoding the primary collagens in valve tissue have not been linked to autosomal dominant MVP.59,60 Multiple single-nucleotide polymorphisms were detected at a higher frequency in people with idiopathic myxoid degeneration than in a control population, but their significance remains unclear.61,62 In a rare related condition, X-linked myxomatous valvular dystrophy, in which all of the valves are thickened, mutations in filamin A, a gene that previously was identified only as a cause of neurologic and skeletal disorders, have been implicated. 63 MVP is not confined to humans. Specific breeds of dog, including the Cavalier King Charles Spaniel and the dachshund, have a high prevalence of prolapse that may lead to severe MR and congestive heart failure later in life.64,65 The disease is associated with dysmorphic leaflets and is inherited, suggesting a strong genetic linkage, although the specific genetic determinants remain to be elucidated. An experimental murine model of Marfan syndrome–associated MVP has been described in which TGF-β signaling is increased as a result of fibrillin-1 deficiency. 66 Interestingly, the mitral valve changes progressed over time. Abnormal signaling involving the TGF-β system has also been postulated as a mechanism of abnormality in X-linked valve dystrophy. 67 Although these animal models provide very useful insights into mechanism of disease, the pathophysiologic mechanisms operating in human idiopathic MVP appear to differ from those in both Marfan syndrome and the X-linked disorder.26,68 The currently accepted prevalence of MVP in the community is based on the Framingham Heart Study. 69 In that population-based study, the echocardiograms of 3491 individuals (1845 men and 1646 women) were reviewed. The mean age of the population was 55 years. MVP was determined on long-axis views of the valve and was seen in 84 subjects or 2.4% of the population. Classic prolapse, in which leaflet thickness greater than 5 mm and leaflet prolapse are present, was seen in 1.3% of those studied; nonclassic prolapse, in which leaflet displacement alone was apparent, occurred in 1.1%. There was no significant gender difference in prevalence, unlike in earlier studies without a strict echocardiographic definition, in which women predominated. Prevalence was 2% to 3% in each decade of age from 30 to 80. The subjects with MVP had a greater likelihood of MR than those without prolapse, with more regurgitation being evident in the group with classic prolapse, who had, on average, mild regurgitation. Those with nonclassic prolapse had, on average, trace MR. Other complications, such as atrial fibrillation, congestive heart failure, cerebrovascular disease, and syncope, were no more common in the MVP group than in the rest of the population studied. MVP has been detected in many population groups of different ethnic and racial backgrounds. 70 In one Canadian study, the prevalences were similar in Caucasian, Indian, and Chinese populations. 71 When MVP is suspected clinically and confirmed by echocardiography, long-term follow-up suggests a more complicated course than that experienced by subjects detected by community screening as in the Framingham study. A Mayo Clinic study identified 833 asymptomatic patients with MVP between 1989 and 1998 in Olmsted County, of whom about two thirds presented with a murmur, and in one third MVP was detected on an echocardiogram performed for another reason. 72 The mean age of this cohort was 47 years. Those presenting with a murmur tended to have more severe MR and a larger left atrium, and were less likely to have atrial fibrillation at the outset. Ten-year mortality for the total cohort was 19% but was not statistically greater than expected. Cardiovascular mortality at 10 years was 9%. Predictors of cardiovascular mortality were moderate or more severe MR and LV ejection fraction less than 50%. Site of prolapse, presence of flail, and LV size did not influence mortality. Cardiovascular morbid events in follow-up occurred in 171 patients. These included heart failure in 60, new-onset atrial fibrillation in 51, ischemic neurologic events in 38, peripheral thromboembolism in 11, endocarditis in 4, and mitral valve surgery in 65 patients. Ten-year cardiovascular morbidity was 30% and was predicted by age 50 or older, left atrial size 40 mm or greater, MR of any severity but higher odds ratio for more severe MR, flail leaflet, and baseline atrial fibrillation. Gender, location of prolapse, LV size, and valve thickening did not independently predict cardiovascular morbidity. Prior studies of patients with MVP also suggested high-risk features detected clinically and on an echocardiogram. In a cohort of 237 asymptomatic or minimally symptomatic patients with MVP studied in the 1980s at the Mayo Clinic who were followed for an average of 6 years, the survival at 8 years was predicted to be 88%, no different from that of a control population. Factors indicative of worse outcome included an end-diastolic LV dimension greater than 6 cm, which was an indicator of a need for mitral valve surgery. Valve redundancy was another strong negative predictor, with sudden death, infective endocarditis, or a cerebral ischemic event occurring in 10% of the 97 patients with this finding, whereas only 1 of 140 patients without redundancy experienced such an event. 12 In another study of 456 patients reported in the late 1980s, the population was classified as having classic MVP on the basis of leaflet thickening and redundancy or nonclassic MVP on the basis of the absence of these features. Complications were more common in the classic group. These included endocarditis in 3.5% of the classic group versus 0% of the nonclassic group, significant MR in 12% and 0%, respectively, and the need for mitral valve surgery in 7% and 1%, respectively. The incidence of stroke was significant, being 6% to 7% in both groups. The age of onset of MVP is variable. One study failed to detect MVP in neonates, even in offspring of affected parents. 73 It appears to be relatively uncommon in pediatric populations except in the setting of a primary disorder of connective tissue such as Marfan syndrome. 74 Symptomatic presentation is most common in midlife, and surgical intervention for severe MR is most likely in the sixth or seventh decade.21,75 In one study, the average time from detection of a murmur to symptomatic presentation was 24 years. 76 Once symptoms occurred, surgical intervention was required within 1 year and the mean age at surgery was 60 years. Thus, by inference, it appears that the mean age at which the murmur was detected was 35 years. It was estimated, in the earlier era of echocardiographic diagnosis of MVP, that approximately 4% of men and 1.5% of women in Australia 77 with the condition would eventually require surgery, whereas the estimates for the United States were 5% and 1.5%, respectively. 78 Given the surfeit of diagnoses of MVP in this era, the likelihood is that with a stricter definition of prolapse a greater proportion of these patients will eventually need surgery. Thus, a later estimate of the need for mitral valve surgery by age 70 is 11% in men with mitral prolapse and 6% for women. 79 Despite the risk of complications, MVP appears to have an excellent prognosis. When patients with severe MR from MVP undergo repair surgery at an appropriate time, there is considerable evidence that their survival is as good as, if not better than, that of a control population without MVP.75,80,81 This is not necessarily true if a mitral valve replacement is performed or if MR has led to LV dysfunction.82–84 The excellent prognosis in reported series of MVP may reflect a lower than expected rate of coronary artery disease in these series either by design (exclusion of patients with both coronary disease and mitral repair in surgical series) or by chance. In the Framingham study, subjects with MVP showed a trend toward a lower prevalence of coronary disease. In one surgical series in which patients who had coronary artery disease and myxomatous disease were compared with those who had ischemic heart disease alone, survival rates were impaired equally in both groups and depended on severity of ischemic heart disease and LV dysfunction. 85 Failure to appropriately intervene surgically in MVP with severe MR does lead to impairment of survival, however, illustrating the importance of careful follow-up of such patients at regular intervals. 86 Referral to surgery before the onset of symptoms or of LV dilation or dysfunction is now indicated by the most recent American College of Cardiology (ACC)/American Heart Association (AHA) guidelines if the likelihood of successful repair is greater than 90%. 87 Most patients presenting with MVP for the first time are asymptomatic, and the diagnosis is made on the basis of the characteristic physical findings or because an echocardiogram is being performed for another reason. Nevertheless, patients with MVP may present with specific symptoms referable to the valve. These include shortness of breath and even heart failure when significant MR is already present. Sudden onset of shortness of breath and heart failure requiring immediate treatment may result from acute chordal rupture or from valve leaflet perforation or valve disruption in endocarditis. 88 Patients with MVP may experience chest pain that is atypical for angina. 89 The mechanism by which this pain occurs is unknown. Palpitations are common in patients with MVP, even in those with little or no MR. 90 Most frequently the palpitations consist of ventricular extrasystoles that may be multifocal or clustered. Atrial extrasystoles are also common. 91 However, in a blinded study of Holter monitor recordings from patients with MVP and from control subjects, no difference in frequency or complexity of rhythm disturbance was noted. 92 Nevertheless, once MR is present, arrhythmia is common and is more frequent in women and with advancing age. 91 Atrial fibrillation and atrial flutter are common later in the course of MVP, when significant MR has been established for some time and atrial enlargement has ensued. 93 Rarely, MVP manifests initially as ventricular tachycardia or sudden death.34,94,95 MVP may also manifest as subacute bacterial endocarditis96,97 with fever and systemic illness or with a stroke or transient ischemic attack.98,99 MVP is reliably diagnosed by both physical examination and 2D echocardiography. Classic physical findings for MVP include a dynamic midsystolic to late systolic click followed by a high-pitched systolic murmur heard at the cardiac apex. With more advanced MR, the murmur may extend throughout systole, and with severe MR or associated LV dysfunction, a third sound may be heard and the click may be inaudible, but the murmur is usually loud.100,101 The click is thought to result from stretching of redundant valve and chordal tissue. A click may occur without any murmur when the leaflets are redundant but not regurgitant. In addition, systolic clicks may arise from other pathologic conditions, including bicuspid aortic valve, atrial myxoma, and pericarditis. A click, therefore, is sensitive but not very specific for the diagnosis of MVP, although a midsystolic click with late systolic murmur is highly likely to represent myxomatous degeneration of the mitral valve. Provocative maneuvers such as the Valsalva maneuver, squatting, and leg raises may improve the diagnostic likelihood of MVP by illustrating that the click moves within systole in response to volume and loading changes. 102 A reduction in end-diastolic volume, such as that occurring with the Valsalva maneuver or standing, causes the click to occur earlier, whereas an increase in end-diastolic volume such as that occurring with squatting or decreasing contractility or increasing afterload (hand-grip) moves the click later in systole. The murmur may radiate on the basis of the direction of the regurgitant leak and the leaflet that is prolapsing. Thus, with anterior leaflet prolapse and a posteriorly directed jet, the murmur may be appreciated very well at the back. Secondary causes of MVP such as Marfan syndrome have specific skeletal and morphologic findings that aid in their identification. These are not seen routinely in idiopathic MVP, which, in contrast, has no specific features other than the cardiac manifestations that render the diagnosis likely. 103 Patients with MVP in the Framingham study were significantly leaner, according to lower body mass index and waist-to-hip ratio, than those without prolapse. 69 Lower weight and lower blood pressure in patients with MVP have been described in other studies. 90 Older studies suggested a higher incidence of skeletal abnormalities associated with MVP, such as straight back and asthenic build.90,104 A 2D echocardiogram is required for precise diagnosis of MVP and to determine the presence of MR and other findings that affect prognosis and risk of complications ( Figure 18-6). Prolapse was detected initially on echocardiography by M-mode echocardiography. The characteristic appearance of late systolic hammocking of the mitral leaflets was used to make the diagnosis. However, apart from demonstrative purposes, M-mode echocardiography has little role in the current diagnosis of prolapse. Usually a transthoracic echocardiogram is adequate for simple diagnostic purposes, although both transesophageal echocardiography (TEE) and three-dimensional (3D) echocardiography may provide specific information that is not available from the transthoracic window. In fact, 3D echocardiography has been most successfully applied to the evaluation of the mitral valve apparatus, providing greater insight into its pathologies as well as the likelihood of and operative plans for successful repair. Stress echocardiography provides powerful additional diagnostic and prognostic information in selected individuals. FIGURE 18-6 A, Parasternal long-axis view of prolapse of the posterior mitral leaflet into the left atrium (LA) (arrow). B, The anteriorly directed regurgitant jet of mitral regurgitation away from the prolapsing leaflet is demonstrated. Ao, aorta; LV, left ventricle. On 2D echocardiography, MVP is diagnosed when either or both of the leaflets are displaced 2 mm or more in systole above a line connecting the annular hinge points in the parasternal or apical long-axis view.105–107 Displacement of the leaflets above this line in other imaging windows, specifically the apical four-chamber window, should not be considered abnormal. In an earlier era, however, prolapse identified on the apical four-chamber view was considered to be diagnostic of true MVP, leading to an epidemic of diagnosis in 38% of teenage girls. 13 Using 3D echocardiography, Levine et al105–107 demonstrated in the late 1980s that the mitral annulus was not planar, but rather had a complex saddle-shaped structure in which the anterior and posterior portions of the annulus are higher than the lateral portions ( Figure 18-7). Thus, in the anteroposterior axis, the annulus is concave upward, whereas in the mediolateral axis, the annulus is concave downward. The result is that in the apical four-chamber plane, even normal leaflets may appear to break the annular plane. Levine et al 105 went on to show that prolapse identified only on the apical four-chamber view did not exhibit the other features of pathologic MVP, such as chamber enlargement and leaflet thickening, and should not be considered abnormal. FIGURE 18-7 Diagrams of saddle-shaped mitral valve annulus. Myxomatous changes in the mitral valve leaflets may lead to thickening of leaflets and chordae and to enlargement of the mitral valve annulus. 108 These are especially evident when MR is present. Thickening of the leaflets to 5 mm or more is considered “classic” for MVP and is predictive of subsequent complications ( Figure 18-8). 109 Mitral valve thickening is measured in diastole from the leading edge to the trailing edge of the thickest area of the midportion of the leaflet and not as the dimension of maximal area of focal leaflet thickening. 69 In many instances, the leaflets and chordae are very thickened and redundant.12,110 It is also common to identify tricuspid valve prolapse in patients with MVP. 111 Tricuspid valve prolapse may occur in up to 40% of all patients with MVP, whereas aortic valve prolapse is much less prevalent and occurs in 1% to 2% of patients with MVP. 87 The thickness of the leaflets changes much more than normally from systole to diastole in MVP on echocardiography. 112 This finding has been attributed to inherent increased thickening of the valve structures that has been demonstrated pathologically and also to increased redundancy of the leaflet tissue. Apical four-chamber views are not useful in detecting mild MVP, but once the diagnosis has been made, this imaging window is very helpful in defining the precise leaflet involvement and the severity of MR. In addition, prolapse of the lateral scallop of the posterior leaflet may be evident only on this view. FIGURE 18-8 Parasternal long-axis echocardiogram of bileaflet prolapse in systole (A) and diastole (B).
Myxomatous Mitral Valve Disease
Definition
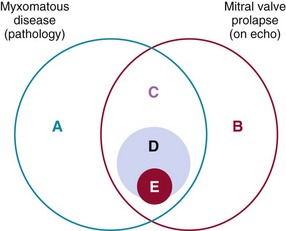
A, Myxomatous disease can exist in a preclinical state before the onset of prolapse. B, Mitral valve prolapse may occur on echocardiography as a benign condition without myxomatous change mainly due to valve-ventricle disproportion. C, Myxomatous valve disease is a major cause of mitral valve prolapse. D, A subset of patients with myxomatous disease and MVP have high-risk clinical and echocardiographic features that make them more prone to the development of complications (E), such as severe mitral regurgitation requiring surgery and endocarditis.
Anatomy of the Normal and Myxomatous Mitral Valve
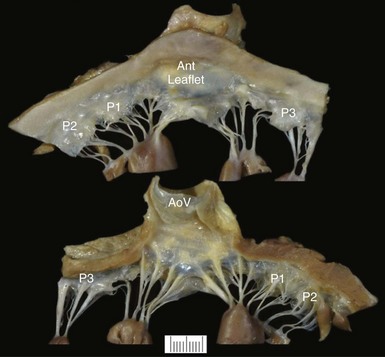
The thin translucent leaflets and chordae and papillary muscles are visualized from the atrial side (top) and the ventricular side (bottom). The two leaflets (Ant, anterior) and the three scallops of the posterior leaflet (P1, P2, and P3) are shown. AoV, Aortic valve.
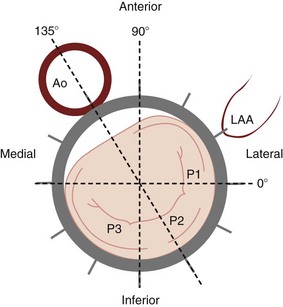
Angles correspond to the transesophageal echocardiography planes that cut the mitral valve as demonstrated. The exact location of the plane depends on the height of the probe in the esophagus and the extent of anteflexion or retroflexion. Ao, Aorta; LAA, left atrial appendage; P, posterior; P1, P2, P3, the three scallops of the posterior leaflet.
Etiology and Pathology of Mitral Valve Prolapse
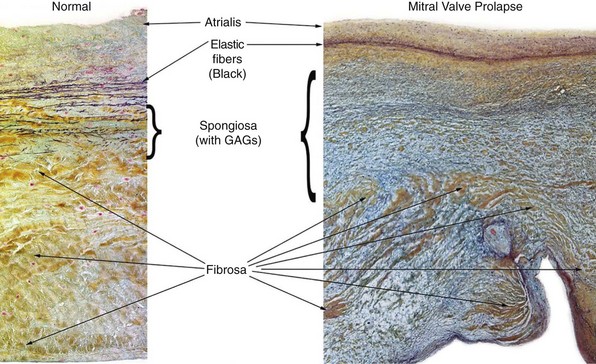
The spongiosa is thicker in mitral valve prolapse than in the normal valve with a greater concentration of glycosaminoglycans (GAGs), some of which extend into the fibrosa. (Courtesy Rene Rodriguez, MD, Department of Cardiovascular Pathology, Cleveland Clinic.)
Genetic Factors
Epidemiology and Natural History
Diagnosis and Clinical Features
Symptoms
Cardiac Physical Findings
Noncardiac Physical Findings
Echocardiographic Diagnosis
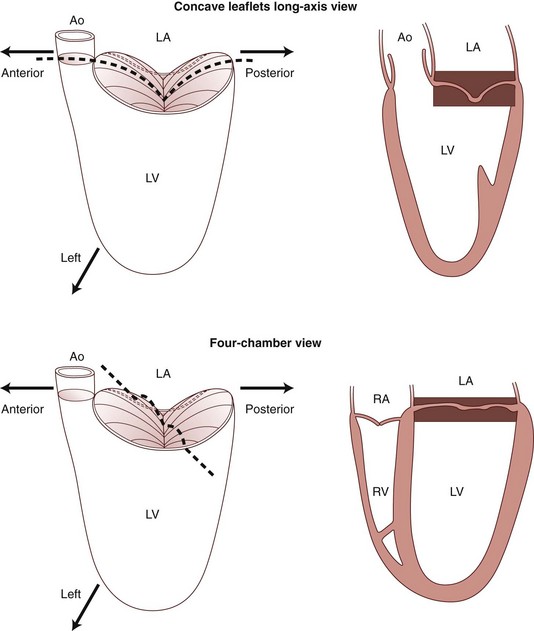
The diagrams of the imaging planes indicate how apparent prolapse may occur on an apical cross-sectional image in the absence of any prolapse in a long-axis cross-section. Ao, Aorta; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. (From Levine RA, Triulzi MO, Harrigan P, et al. The relationship of mitral annular shape to the diagnosis of mitral valve prolapse. Circulation 1987;75:756–67.)
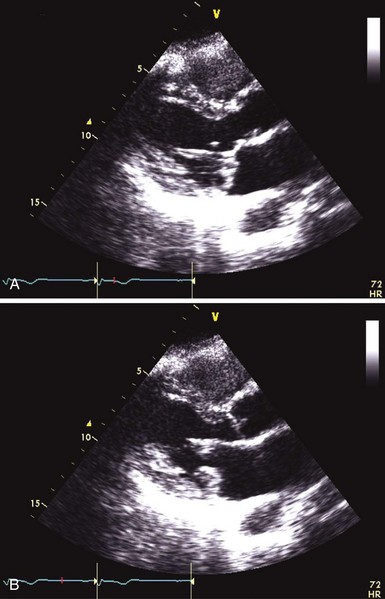
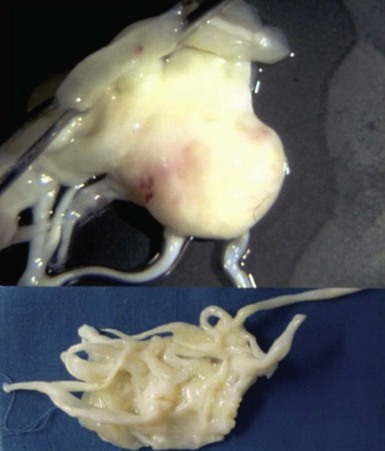
Significant thickening and redundancy of the leaflets in diastole can be seen, consistent with “classic” mitral valve prolapse.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Myxomatous Mitral Valve Disease
Only gold members can continue reading. Log In or Register to continue





