Treatment for severe symptomatic aortic stenosis has changed significantly in recent years due to advances in transcatheter aortic valve replacement (TAVR). Recent studies with the CoreValve prosthesis have demonstrated superior results compared with surgical aortic valve replacement in patients at increased risk for surgery, but there are limited data on gender-related differences in patient characteristics and outcomes with this device. We compared baseline characteristics and clinical outcomes in women and men undergoing TAVR with the CoreValve prosthesis. A total of 3,687 patients (1,708 women and 1,979 men) were included. At baseline, women tended to be slightly older and to have increased frailty, but they had fewer cardiac co-morbidities, higher left ventricular systolic function, less coronary artery disease, and fewer previous strokes. All-cause mortality was 5.9% for women and 5.8% for men at 30 days (p = 0.87) and 24.1% and 21.3%, respectively, at 1 year (p = 0.08). The incidence of stroke was 5.7% in women and 4.0% in men at 30 days (p = 0.02) and 9.3% and 7.7%, respectively, at 1 year (p = 0.05). Women had a higher incidence of bleeding, including more life-threatening bleeds, and a greater incidence of major vascular complications than men at 30 days. Device success was achieved in 86.9% of women and 86.1% of men (p = 0.50). In conclusion, although there were significant baseline differences and procedure-related complications between women and men undergoing TAVR with the CoreValve prosthesis, this analysis found no significant difference in 30-day or 1-year mortality.
The treatment paradigm for severe symptomatic aortic stenosis has changed significantly in the past several years due to advances in transcatheter aortic valve replacement (TAVR). Recent studies with the CoreValve prosthesis (Medtronic, Minneapolis, Minnesota) have demonstrated superior results compared with surgical aortic valve replacement (SAVR) in patients at increased risk for surgery. There are limited data, especially from the United States, on gender-related differences in patient characteristics and outcomes with the CoreValve device. Furthermore, unlike percutaneous coronary intervention, which men undergo much more frequently than women, approximately half of all US TAVR patients are women. CoreValve registry data from Europe have shown similar early and midterm outcomes between men and women with a possible survival benefit in women with longer follow-up ; however, a rigorous, prospective evaluation of this device in women has not been performed. Studies of the balloon-expanding aortic valve have suggested that the mortality benefit of TAVR over SAVR is greater for women at 1 year and that female gender is associated with better short- and long-term outcomes in patients at high risk.
Methods
The objective of this study was to compare the baseline characteristics and clinical outcomes in women and men undergoing TAVR with the CoreValve prosthesis in the United States. The analysis included all patients enrolled in the CoreValve US Pivotal Trials and Continued Access Study in whom TAVR was attempted. These trials included patients at extreme and high risk for surgical valve replacement. The Continued Access Study began enrollment after enrollment in the Pivotal Trials was complete, using the same inclusion and exclusion criteria, until commercialization. The trials were conducted at 45 US sites.
An independent Clinical Events Committee adjudicated all major adverse clinical events. The Data and Safety Monitoring Board was responsible for study oversight.
Eligibility criteria included severe symptomatic aortic stenosis with a New York Heart Association (NYHA) functional status of class II or greater. Severe aortic stenosis was defined as an aortic valve area ≤0.8 cm 2 or an aortic valve index ≤0.5 cm 2 /m 2 and either a mean aortic valve gradient >40 mm Hg or a peak aortic valve velocity >4.0 m/s at rest or with a dobutamine stress if the left ventricular ejection fraction was <50%. Patients were considered high risk if 2 cardiac surgeons and 1 interventional cardiologist at the clinical site estimated a 15% or greater risk of mortality at 30 days with a risk of mortality or irreversible complications of <50%. Patients were considered extreme risk if the risk of mortality or irreversible complications was ≥50% at 30 days.
A case summary that included co-morbidities and site-reported imaging findings was created for each patient and was presented by the clinical site heart team to a National Screening Committee. At least, 2 senior cardiac surgeons and 1 interventional cardiologist had to agree that the patient met study eligibility, risk, and imaging criteria for the trial. Screening imaging included not only 2-dimensional transthoracic echocardiography but also computed tomography angiography with complete visualization of both iliac and femoral arteries and the aorta (and subclavian/axillaries if applicable) up to and including the aortic annulus.
The CoreValve prosthesis consists of a self-expanding nitinol frame that supports a trileaflet porcine pericardial valve. The valves available in the CoreValve US trials included those with 23-, 26-, 29-, and 31-mm diameters and were used for treating patients with an annulus ranging from 18 to 29 mm in diameter. All valve sizes are loaded into the delivery catheter outside the body before the procedure and are then advanced through an 18F sheath and deployed without rapid ventricular pacing.
A detailed assessment of the patient baseline co-morbidities was performed using the Society for Thoracic Surgery Predicted Risk of Mortality, EuroSCORE, and Charlson Co-morbidity Index. Frailty markers included a 5-m gait speed test and grip-strength testing. Disability was assessed using the Katz Index of Activities of Daily Living and the Mini-Mental State Examination for dementia.
The primary end point in the high-risk pivotal and high-risk continued access trials was the rate of all-cause mortality 12 months after the procedure. All-cause mortality or major stroke at 12 months was the primary end point in the extreme-risk pivotal and continued access studies. Major and minor stroke were defined using Valve Academic Research Consortium (VARC)-1 criteria. Criteria for major adverse cardiovascular and cerebral events, comprising all-cause death, myocardial infarction, all stroke, and reintervention to alter, adjust, or replace a previously implanted valve, along with additional secondary end points have been previously published. Symptom status was assessed using the NYHA classification system. Device success was defined using the VARC-1 criteria. Procedure success was defined as device success in the absence of in-hospital major adverse cardiovascular and cerebral events.
Serial echocardiograms were obtained at screening, after the procedure (within 24 to 48 hours), hospital discharge, and 1, 6, and 12 months after valve implantation and were interpreted by personnel at each site. Prosthetic valve dysfunction and periprocedural aortic regurgitation were determined using VARC-1 criteria.
Categorical variables were compared using the chi-square or Fisher’s exact test. Continuous variables were presented as mean ± SD and compared using the Student t test. Kaplan–Meier event estimates were used to construct the survival curves based on all available follow-up data for the time-to-event analysis. The log-rank test was used to compare the time to events. All testing used a 2-sided α level of 0.05. All statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, North Carolina).
Results
Of a total of 3,687 patients, women made up 46.3% (n = 1,708) of the cohort. In all, 1,089 women were enrolled in the extreme-risk cohorts (63.8%) and 619 in high-risk cohorts (36.2%), and 1,173 men were enrolled in the extreme-risk cohorts (59.3%) and 806 in the high-risk cohorts (40.7%). Women tended to be slightly older with a higher STS score but a lower logistic EuroSCORE. Although there was no difference between women and men in baseline NYHA classification, women tended to have fewer cardiac co-morbidities than men and higher left ventricular systolic function at baseline ( Table 1 ). Among women, there was a lower rate of coronary artery disease, including fewer myocardial infarctions, percutaneous coronary interventions, and coronary artery bypass surgeries ( Table 1 ). Women also were less likely to have had a previous stroke, have peripheral vascular disease, or have a preexisting pacemaker or defibrillator ( Table 1 ).
| Demographics | Men (N=1979) | Women (N=1708) | P |
|---|---|---|---|
| Age (years) | 82.7±7.9 | 84.0±7.6 | <0.01 |
| New York Heart Association class III/IV | 1712 (86.5%) | 1506 (88.2%) | 0.13 |
| Society for Thoracic Surgery Predicted Risk of Mortality score (%) | 8.3±4.6 | 9.6±4.9 | <0.01 |
| Logistic European System for Cardiac Operative Risk Evaluation score (%) | 23.4±16.5 | 20.7±15.0 | <0.01 |
| Left ventricular ejection fraction (%) | 51.0±14.3 | 57.7±12.3 | <0.01 |
| Coronary artery disease | 1756 (88.7%) | 1155 (67.6%) | <0.01 |
| Previous myocardial infarction | 680 (34.4%) | 336 (19.7%) | <0.01 |
| Prior coronary artery bypass surgery | 989 (50.0%) | 309 (18.1%) | <0.01 |
| Prior percutaneous coronary intervention | 874 (44.2%) | 532 (31.1%) | <0.01 |
| Prior balloon valvuloplasty | 246 (12.4%) | 229 (13.4%) | 0.38 |
| Diabetes mellitus | 784 (39.6%) | 601 (35.2%) | <0.01 |
| Chronic kidney disease class 4/5 | 161 (8.2%) | 283 (16.7%) | <0.01 |
| History of hypertension | 1836 (92.8%) | 1582 (92.6%) | 0.86 |
| Prior stroke | 295 (14.9%) | 191 (11.2%) | <0.01 |
| Prior transient ischemic attack | 218 (11.0%) | 176 (10.3%) | 0.49 |
| Peripheral vascular disease | 1011 (51.3%) | 664 (39.0%) | <0.01 |
| Pre-existing pacemaker/defibrillator | 525 (26.5%) | 308 (18.0%) | <0.01 |
| Prior atrial fibrillation/atrial flutter | 927 (47.0%) | 699 (41.0%) | <0.01 |
| Society for Thoracic Surgery lung disease (moderate/severe) | 692 (35.0%) | 554 (32.4%) | 0.10 |
| Home oxygen | 406 (20.5%) | 404 (23.7%) | 0.02 |
Evaluation of frailty indexes demonstrated that women tended to be more frail and had more physical limitations than men. Women had a higher incidence of being wheelchair bound, a greater number of deficits in Katz activities of daily living, a higher incidence of low body mass index, and a slower 5-m gait speed. There was no difference in baseline albumin between women and men ( Table 2 ).
| Assessment | Men (N=1979) | Women (N=1708) | P |
|---|---|---|---|
| Wheelchair bound | 125 (6.3%) | 170 (10.0%) | <0.01 |
| Katz activities of daily living | |||
| ≥2 Deficits | 158 (8.0%) | 223 (13.1%) | <0.01 |
| ≥3 Deficits | 96 (4.9%) | 135 (7.9%) | <0.01 |
| Body mass index <21 kg/m 2 | 115 (5.8%) | 196 (11.5%) | <0.01 |
| Albumin <3.3 g/dL | 338 (17.3%) | 277 (16.5%) | 0.51 |
| 5-m gait speed (seconds) | 9.4±10.7 | 12.1±15.0 | <0.01 |
| Grip strength below threshold | 1275 (65.2%) | 1214 (72.0%) | <0.01 |
Women were slightly more likely than men to have implantation performed through an alternative (noniliofemoral) access route ( Table 3 ). Among patients undergoing TAVR using an alternative access, women were more likely than men to have a direct aortic approach used and less likely to have a subclavian approach used ( Table 3 ). Valve sizing showed that women on average tended to have smaller valves implanted than men ( Table 3 ). Conversion to surgery was extremely low for both groups with no statistical difference between the 2 genders; however, men were more likely to require placement of a second valve. Device success (VARC-1 criteria) was no different between men and women, and there was no significant difference in moderate or severe paravalvular aortic insufficiency as measured by postprocedure transthoracic echocardiography 24 to 48 hours after TAVR ( Table 3 ).
| Variable | Men (N=1979) | Women (N=1708) | P |
|---|---|---|---|
| Access approach | |||
| Iliofemoral | 1614/1975 (81.7%) | 1346/1703 (79.0%) | 0.04 |
| Non-iliofemoral | 361/1975 (18.3%) | 357/1703 (21.0%) | 0.04 |
| Subclavian | 137/1975 (6.9%) | 78/1703 (4.6%) | <0.01 |
| Direct aortic | 224/1975 (11.3%) | 279/1703 (16.4%) | <0.01 |
| Valve size (mm) | |||
| 23 | 0/1967 (0.0%) | 59/1695 (3.5%) | <0.01 |
| 26 | 84/1967 (4.3%) | 829/1695 (48.9%) | <0.01 |
| 29 | 922/1967 (46.9%) | 723/1695 (42.7%) | 0.01 |
| 31 | 961/1967 (48.9%) | 84/1695 (5.0%) | <0.01 |
| Procedure results | |||
| >1 valve implanted | 106 (5.4%) | 55 (3.2%) | <0.01 |
| Conversion to surgery | 2/1974 (0.1%) | 3/1697 (0.2%) | 0.67 |
| Device success | 1654/1921 (86.1%) | 1423/1638 (86.9%) | 0.50 |
| Successful vascular access, delivery, and deployment of the device | 1942/1974 (98.4%) | 1667/1696 (98.3%) | 0.83 |
| No moderate or severe aortic insufficiency 24-48 h after implantation | 1864/1958 (95.2%) | 1611/1670 (96.5%) | 0.06 |
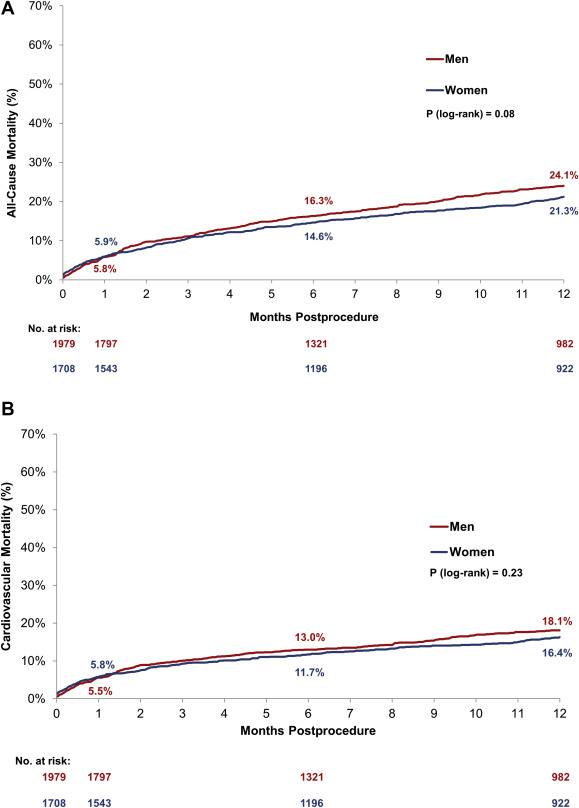
All-cause mortality for women and men was similar at 30 days, and there remained no significant difference by 1 year ( Figure 1 and Table 4 ). There was also no difference in cardiovascular mortality from baseline to 30 days or 1 year ( Figure 1 and Table 4 ). Women had a higher incidence of bleeding, including more life-threatening bleeds, as well as an increased incidence of major vascular complications and cardiac tamponade compared with men at 30 days. In addition, there was a greater incidence of major stroke in women compared with men from baseline to 30 days that remained significant out to 1 year ( Table 4 ). Fewer women than men required the placement of a permanent pacemaker in the postprocedure period out to 30 days ( Table 4 ). Both women and men had a >22-point average improvement in their Kansas City Cardiomyopathy Questionnaire score from baseline to 30 days ( Table 4 ).
| 30 days | 1 year | |||||
|---|---|---|---|---|---|---|
| Men (N=1979) | Women (N=1708) | P | Men (N=1979) | Women (N=1708) | P | |
| All-cause mortality | 114 (5.8%) | 100 (5.9%) | 0.87 | 406 (24.1%) | 315 (21.3%) | 0.08 |
| Cardiovascular | 109 (5.6%) | 98 (5.8%) | 0.74 | 305 (18.1%) | 242 (16.4%) | 0.23 |
| Stroke | 79 (4.0%) | 95 (5.7%) | 0.02 | 129 (7.7%) | 141 (9.3%) | 0.05 |
| Major | 42 (2.1%) | 60 (3.6%) | 0.01 | 72 (4.5%) | 86 (5.6%) | 0.04 |
| Minor | 37 (1.9%) | 38 (2.3%) | 0.44 | 59 (3.4%) | 61 (4.2%) | 0.34 |
| All-cause mortality or major stroke | 142 (7.2%) | 140 (8.2%) | 0.22 | 434 (25.6%) | 353 (23.4%) | 0.32 |
| Bleed | 615 (31.2%) | 728 (42.7%) | <0.01 | 694 (36.7%) | 781 (46.8%) | <0.01 |
| Life-threatening/disabling | 200 (10.2%) | 244 (14.3%) | <0.01 | 261 (14.3%) | 290 (17.9%) | <0.01 |
| Major | 427 (21.7%) | 504 (29.7%) | <0.01 | 477 (25.4%) | 535 (32.1%) | <0.01 |
| Major vascular complication | 96 (4.9%) | 165 (9.7%) | <0.01 | 103 (5.3%) | 168 (9.9%) | <0.01 |
| Acute kidney injury | 222 (11.3%) | 175 (10.4%) | 0.38 | 222 (11.3%) | 175 (10.4%) | 0.38 |
| Myocardial infarction | 16 (0.8%) | 18 (1.1%) | 0.43 | 33 (2.1%) | 34 (2.4%) | 0.52 |
| Cardiac tamponade | 11 (0.6%) | 42 (2.5%) | <0.01 | 15 (0.8%) | 43 (2.5%) | <0.01 |
| New permanent pacemaker implant | 453 (23.2%) | 311 (18.6%) | <0.01 | 508 (27.0%) | 346 (21.4%) | <0.01 |
| Kansas City Cardiomyopathy Questionnaire overall summary score change from baseline, mean ± standard deviation | 22.4±27.4 | 22.4±27.2 | 0.96 | 28.0±28.1 | 28.9±26.4 | 0.54 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


