CHAPTER 8 Tracheal Lesions
HISTORICAL PERSPECTIVE
When the field of tracheal surgery was young, it was widely accepted that only four tracheal rings, or approximately 2 cm, of trachea could be safely resected with immediate reconstruction. It was the work of Dr. Hermes Grillo, considered by most to be the father of modern tracheal surgery, that led to the surgical practices used today. Grillo and colleagues1–3 systemically investigated the limits of resection of the trachea to permit reconstruction without excessive anastomotic tension. Among other findings, these studies demonstrated that the tracheal blood supply entered in its lateral pedicles, and that safe mobilization of the trachea could be accomplished only with anterior and posterior dissection. Dedo and Fishman4 described laryngeal release maneuvers to minimize tension on lengthier tracheal resections. These contributions led to the realization that, in select cases, up to 50% of the human trachea could be resected and reconstructed without detrimental tension.
ANATOMY
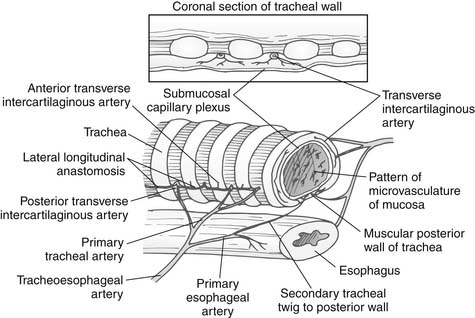
The blood supply to the trachea is from many small terminal end arteries. The upper trachea is supplied principally by branches of the inferior thyroid artery, and the lower trachea by branches of the bronchial arteries. These vessels enter the trachea via very fine lateral pedicles that lack collateralization (Fig. 8-1).5 The pretracheal plane and the plane between the esophagus and the trachea are avascular. The recurrent laryngeal nerves ascend in the tracheoesophageal grooves bilaterally and pass medially to the inferior cornua of the thyroid cartilage to enter the larynx. The nerve on the left is present along the entire length of the trachea, whereas the right recurrent laryngeal nerve is present in the tracheoesophageal groove for only the proximal cervical trachea.
TRACHEAL PATHOLOGY
A wide spectrum of pathology may afflict the trachea, ranging from benign conditions to malignant ones. Most conditions lead to central airway obstruction with subsequent respiratory insufficiency. Others, such as fistulas between the trachea and innominate artery or esophagus, may also occur and be equally deleterious. Direct external trauma, blunt or penetrating, may result in a tear or complete disruption of the trachea at any level. Inhalational burn injuries are usually maximal in the proximal subglottic region and diminish in the more distal airway. In most cases, the tracheal rings are not destroyed.6
Postintubation Injuries
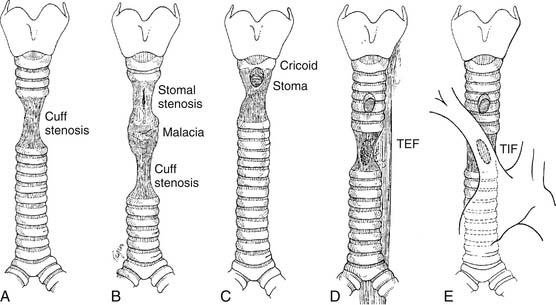
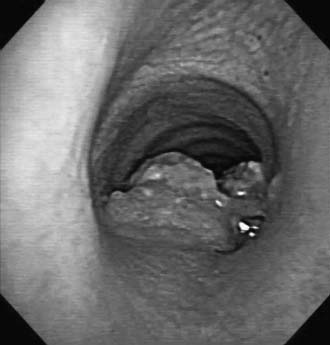
Iatrogenic injuries resulting from tracheal intubation have long been the most common lesion afflicting the airway. Postintubation injuries include granulomas, strictures, malacia, and tracheoesophageal and tracheoinnominate fistulas (Fig. 8-2).
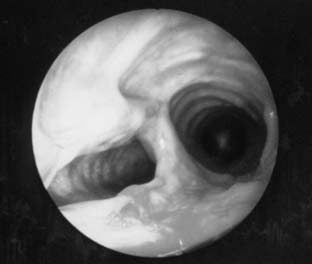
Postintubation tracheal stenosis represents the most common iatrogenic injury of the trachea. It occurs after intubation and develops principally at the level of the endotracheal tube or tracheostomy tube cuffs. The radial pressure exerted from the cuff causes circumferential pressure necrosis, which results in cicatricial scaring stenosis (Fig. 8-3).7,8 The route of entry of the ventilatory tube does not affect the occurrence of cuff injury, only its level. These lesions are seen in patients who have had only oral endotracheal intubation as well as in those with previous tracheostomy. The development of the large-volume, low-pressure cuff for tracheostomy and endotracheal tubes for ventilation has greatly lowered the incidence of cuff stenoses. However, overinflation of these cuffs can result in local airway damage and subsequent scar formation, and it contributes to the continued incidence of these lesions. Stenosis in the subglottic region may occur as a result of prolonged intubation with endotracheal tubes, after cricothyroidotomy, or after high placement of a tracheostomy where the tube erodes through the cricoid cartilage.9
Stomal stenosis results from the gradual enlargement of a tracheal stoma and its eventual healing by contraction. This pulls the sides of the defect together, distorting the tracheal lumen to a triangular configuration, with the base of the triangle located posteriorly and consisting of the uninjured membranous wall (Fig. 8-4). The instigating large stoma may result from overzealous excision of anterior tracheal cartilage at the time of the initial tracheostomy, from erosive infection around the margins of the stoma, or, most commonly, from erosion resulting from leverage by equipment attached to the tracheostomy tube during ventilation. The stenotic process may extend into the subglottic larynx if the previous tracheostomy was placed inappropriately high in the trachea.
Prolonged endotracheal intubation combined with a nasogastric tube may lead to a tracheoesophageal fistula (Fig. 8-5). This results from pressure necrosis generated by a ventilating cuff in the trachea and a prolonged feeding tube in the esophagus. Consequently, these fistulas occur in the shared location between the membranous tracheal wall and the anterior wall of the esophagus. Anterior injuries of the trachea may lead to the development of a tracheoinnominate artery fistula. These most often result from direct erosion of the inner elbow of the tracheostomy tube in an inferiorly placed stoma, and they may arise either from a tracheostomy tube placed too low in the trachea or from an innominate artery lying high at the sternal notch.
Idiopathic Laryngotracheal Stenosis
It is impossible to predict the future course of the disease. When airway obstruction becomes severe and does not respond for sufficiently long intervals to dilation, surgery may be entertained, with, however, the clear caveat that the disease may progress. Most patients are best treated with definitive laryngotracheal resection and primary reconstruction.10,11
Tracheobronchomalacia
Airway malacia may be a consequence of a postintubation injury. However, in patients with chronic obstructive pulmonary disease, malacia may develop in the lower trachea, main bronchi, and sometimes the more distal bronchi, even in the absence of prior intubation.12 When the patient attempts a deep expiration or a cough, the membranous wall approximates to the anterior, softened cartilaginous wall, causing nearly total obstruction. Subsequently, the posterior membranous wall elongates and becomes redundant. Afflicted patients may present with dyspnea, cough, and secretion retention. It is possible to surgically address the deformity by pulling the ends of the cartilages toward one another posteriorly, restoring a more circular shape to the airway. The redundant membranous wall must be tacked to a posterior splinting material to prevent it from falling forward into the lumen. Various materials have been used for splinting, none with complete success. These have included fascia lata, pericardium, lyophilized bone, polytetrafluoroethylene, and rigid plastic splints. We currently use sheets of polypropylene mesh to plicate the membranous wall as described by Rainer and colleagues.13
Tracheal Tumors
Primary tracheal tumors are rare, with an estimated incidence of 2.7 cases per million per year.14 Approximately two thirds of primary tracheal tumors are either squamous cell carcinomas (SCCs) or adenoid cystic carcinomas (ACCs). These two types occur with equal frequency. The remaining third of the tumors are widely distributed in a heterogeneous group of tumors, both malignant and benign. Secondary tumors that involve the trachea include carcinomas of the larynx, thyroid, lung, and esophagus. Rarely, tumors may metastasize to the submucosa of the trachea or to the mediastinum, with secondary invasion of the trachea.
Squamous cell carcinomas may be either exophytic (Fig. 8-6) or ulcerative. The tumor can metastasize to the regional lymph nodes, and, in its more aggressive and late forms, it invades mediastinal structures. In general, its progress appears to be relatively rapid in comparison with that of adenoid cystic carcinoma. A number of these patients develop synchronous or metachronous second SCC primary cancers of the lung or oropharynx. Squamous cell carcinoma occurs predominantly in men who are cigarette smokers.15
ACC often has a very prolonged course of clinical symptoms, sometimes extending for years. After resection, many years may pass before a recurrence is noted. It may extend submucosally over long distances in the airways, and also along perineural tissue. It can spread to regional lymph nodes, although this is less characteristic than in SCC. Although it may invade the thyroid or the muscular coats of the esophagus by contiguity, ACC that has not been surgically interfered with frequently displaces mediastinal structures before actually invading them. Metastases to the lungs, bone, and other organs can occur. These may grow very slowly over a period of many years and remain asymptomatic until they become quite large. In contrast to patients with tracheal SCC, the male-to-female ratio of ACC is essentially equal, and any smoking history of these patients appears to be incidental.15
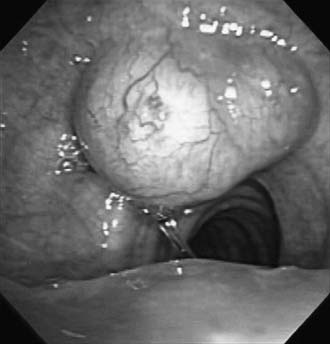
Secondary neoplasms may involve the trachea through direct extension. Thyroid carcinomas typically invade the trachea at the second and third rings, where the thyroid isthmus is adherent to the trachea (Fig. 8-7).16 More commonly, invasion is seen after thyroidectomy for carcinoma, when the surgeon was aware of shaving off the tumor from the trachea. In such cases, concurrent or early resection of the involved trachea should be considered. Both bronchogenic and esophageal carcinomas can erode into the trachea from local extension.
CLINICAL PRESENTATION
Tumors of the trachea often present insidiously. Their most common signs and symptoms are cough (37%), hemoptysis (41%), and the signs of progressive airway obstruction, including shortness of breath on exertion (54%), wheezing and stridor (35%) and, less commonly, dysphagia or hoarseness (7%).14 Signs and symptoms may vary with the histology of the tumor. Hemoptysis is prominent in patients with SCC, usually leading to a prompt diagnosis. Patients with ACC more commonly present with wheezing or stridor as the predominant symptom, which often leads to a delay in diagnosis. In one study, the mean duration of symptoms before diagnosis in patients with SCC of the trachea was only 4 months whereas in those with ACC it was 18 months.17
RADIOGRAPHIC IMAGING
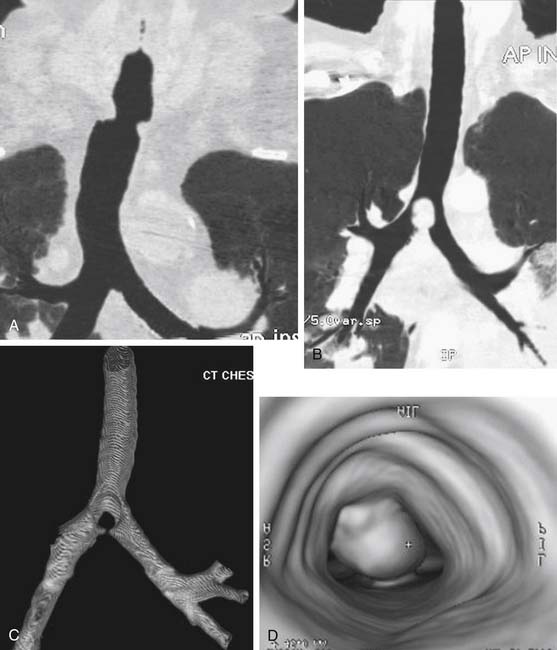
A standard posteroanterior chest radiograph, centered high on the trachea, may reveal some tracheal pathology. Detailed information about the location of the lesion, its longitudinal extent, and the amount of normal trachea available for reconstruction can be demonstrated by computerized axial tomography scans. These are particularly useful in evaluating tracheal tumors, as they characterize extraluminal extension and any mediastinal lymphadenopathy. Recently, the use of high-speed helical computed tomographic scanners to acquire images, combined with powerful three-dimensional-image software, has created impressive two- and three-dimensional airway reconstructions (Fig. 8-8).
BRONCHOSCOPY
Expertise with the techniques of interventional bronchoscopy is essential to safely dilate a benign stenosis or “core out” an obstructing airway tumor. This allows evaluation of the distal airway, safe passage of an endotracheal tube, or temporary creation of a patent airway to allow delay in operation. Dilating a narrow, fibrotic stricture is challenging and can result in airway rupture, complete obstruction, or excessive destruction of tracheal mucosa. Progressively larger Jackson dilators passed through a rigid bronchoscope can be used to effectively dilate the stenosis. An assortment of pediatric and adult rigid bronchoscopes can then be used, increasing the sizes and using a gentle corkscrew motion.18 Balloon dilators placed through the working channel of a flexible bronchoscope permit effective airway dilation.
Obstructing tracheal tumors are managed first by core-out techniques using the rigid bronchoscope, forceps, and suction.19 Using the tip of the bronchoscope in a corkscrew motion, most tumors can be débrided from the tracheal wall, and forceps can then be used to remove dislodged tumor fragments. If bleeding ensues, the bronchoscope is advanced distal to the lesion and used to tamponade the bleeding. Direct application of epinephrine-soaked pledgets can assist in addressing persistent blood loss.
Patients with critical airway stenosis should be assessed in the operating room where rigid bronchoscopy, biopsy forceps, and instruments required to perform emergent tracheotomy are available. Placement of a tracheostomy tube may be necessary in some patients as the only means to secure an airway. When possible, these should be placed through the stenosis, preserving the uninvolved trachea for future reconstruction.18