Fig. 6.1
The pericardium is opened longitudinally anteriorly to the phrenic nerve
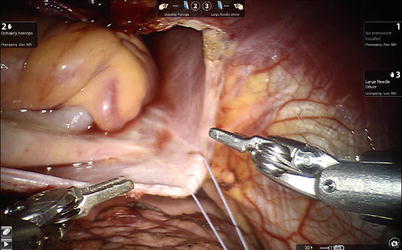
Fig. 6.2
Vertical stay sutures placed on the right side of pericardium
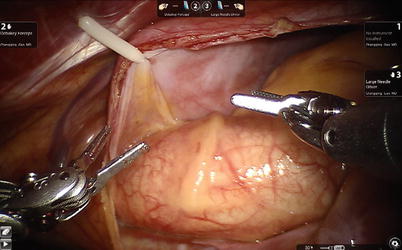
Fig. 6.3
Stay suture on the left superior side of pericardium to expose the ascending aorta
The aortic is occluded with a Chitwood cross-clamp in the fourth ICS via the midaxillary line (Fig. 6.4). Antegrade cold blood cardioplegic solution is administered directly through the anterior chest (the 2nd ICS) with a 14-F angiocatheter (Figs. 6.5 and 6.6).
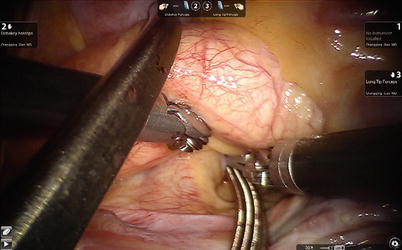
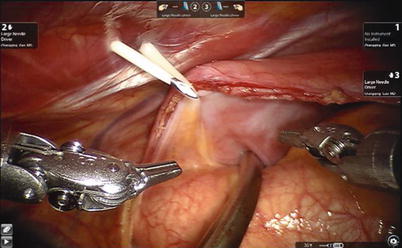
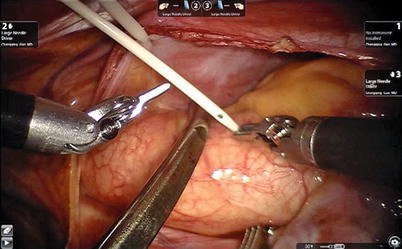

Fig. 6.4
The Chitwood cross-clamp is put in the ascending aorta

Fig. 6.5
The 14-F angiocatheter is punctured into the thoracic cavity on the second ICS in the anterior chest

Fig. 6.6
The 14-F angiocatheter is punctured into the aorta for cardioplegic solution administration
A left atriotomy anterior to the pulmonary veins is performed (Fig. 6.7) and exposure of left atrial myxomas is maximized with the atrial retractor (Figs. 6.8, 6.9, and 6.10).
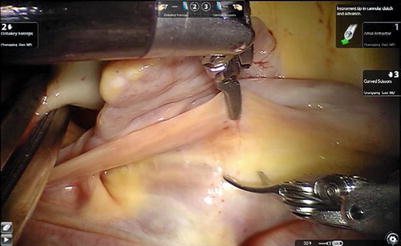
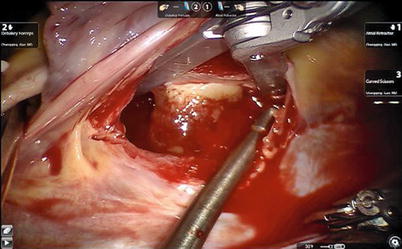
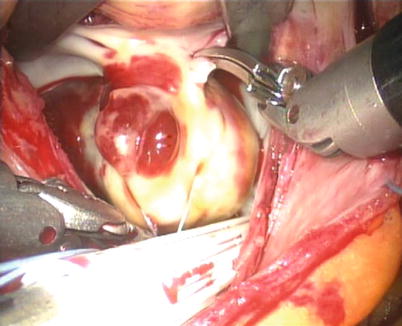
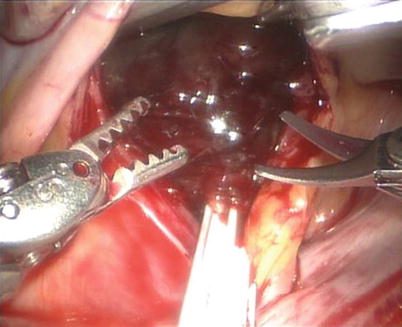

Fig. 6.7
The left atriotomy anterior to the pulmonary veins for the left atrial myxoma

Fig. 6.8
Exposing the left atrial myxoma with atrial retractor

Fig. 6.9
The papillar myxoma in the left atrium

Fig. 6.10
The villous myxoma in the left atrium
For left atrial tumors, the vena cavae are not taped, allowing an increased mobility and exposing the left atrial cavity. The left atriotomy can be extended behind both cavae for greater exposure. Total excision is achieved by dissecting a plane through the atrial muscle at the point of attachment (Figs. 6.11 and 6.12).
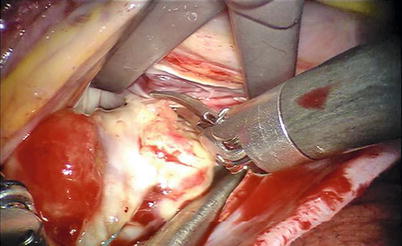
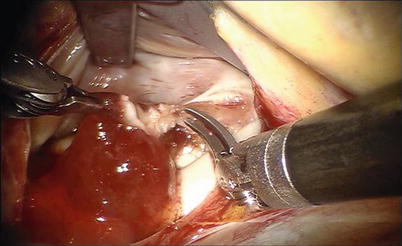

Fig. 6.11
Total excision by dissecting a plane through the atrial muscle at the point of attachment

Fig. 6.12
Total excision of left myxoma
The subendocardial defects are also directly closed with 4-0 polytetrafluoroethylene running suture without pericardial patches (Figs. 6.13 and 6.14). The atrial septal defects originated from the resection can be repaired with autologous pericardial patches with 4-0 polytetrafluoroethylene running suture. Four right atrial myxomas are completely resected from the beating heart with the superior and inferior venae cavae snared avoiding the aorta clamp and cardioplegia administration (Figs. 6.15 and 6.16).
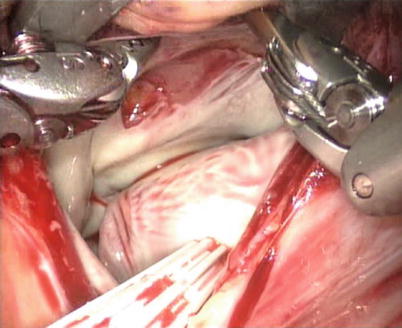
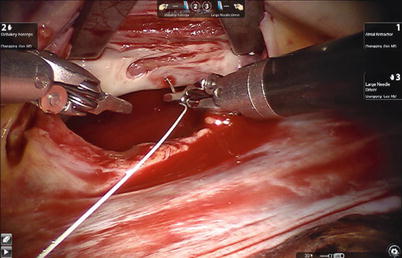
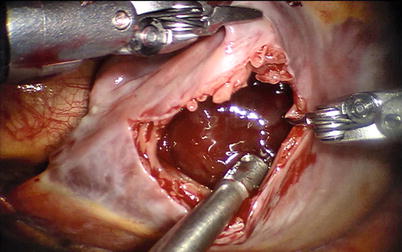
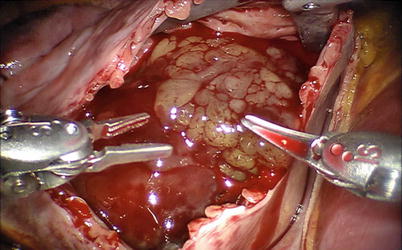

Fig. 6.13
The attachment of myxoma after total excision

Fig. 6.14
Suturing the subendocardial defects

Fig. 6.15
The right atrial myxoma is resected on the beating heart

Fig. 6.16
The right atrial myxoma is exposed with the aid of atrial retractor
Right atrial myxomas pose special venous cannulation problems. Intraoperative echocardiography may be of benefit in allowing safe cannulation. Robotic surgery is constantly safer for right atrial myxomas than sternotomy because of cannulation of the jugular or femoral vein. The tricuspid valve and the right atrium should be inspected carefully in patients with right atrial myxoma, with or without familial myxoma. Regardless of the surgical approach, the ideal resection compasses the tumor and a portion of the cardiac wall or interatrial septum to which it is attached. It is controversial whether excision of full-thickness wall is necessary or excision of only an endocardial attachment is sufficient to prevent recurrence. Our policy is to resect full thickness whenever possible. However, only partial-thickness resection of the area of tumor attachment has been performed when anatomically necessary without a noted increase in recurrence rate [12]. Every care should be taken to remove the tumor without fragmentation. When the tumor is removed from the field, the area should be liberally irrigated, suctioned, and inspected.
The atrial is closed with running sutures after total resection. For excision in arrested heart, after crossclamp release and meticulous intracardiac deairing by angiocatheter of antegrade cold blood cardioplegic solution, the patient is weaned from CPB. After removal of the cardioplegia angiocatheter, the cardioplegia site is closed with extracorporeal knot tying through the working port (Figs. 6.17 and 6.18), and then chest tubes are inserted. The surgical result and integrity of the septal closure are confirmed by TEE (Figs. 6.19 and 6.20).
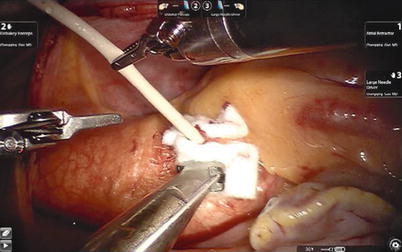
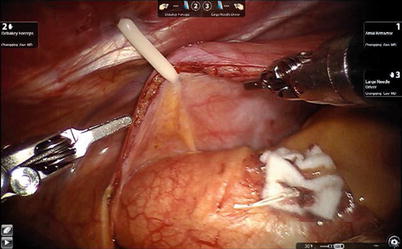
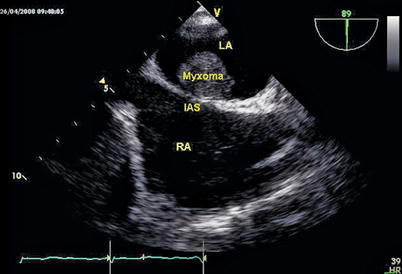
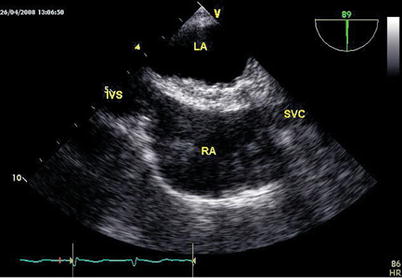

Fig. 6.17
The cardioplegia site is closed with extracorporeal knot tying

Fig. 6.18
The closed cardioplegia site

Fig. 6.19
The pre-operative TEE shows the left atrial myxoma

Fig. 6.20
The surgical resection and integrity of the septal closure are confirmed by TEE
6.3 Postoperative Management
After the operation, patients are monitored at the ICU and discharged to an intermediate care unit as soon as hemodynamics and spontaneous respiration have been adequately stabilized. Chest drains are removed when drainage reaches less than 50 mL/12 h. All patients undergo transthoracic echocardiography immediately before discharge from hospital and at 3 months after the procedure.
6.4 Surgical Experience and Learning Curves
Between July 2007 and May 2013, robotic myxoma resection with da Vinci S or Si Surgical System was performed on 45 consecutive patients at the authors’ hospital. Twelve patients were male and 33 female. The mean age was 47.0 ± 14.1 years old (ranging from 13 to 66). Two patients had preoperative cerebral infarction. The mean tumor size was 43 × 52 mm (14 × 19–44 × 74 mm). Four tumors were found in the right atrium and 36 tumors were found in the left atrium, of which 31 tumors arose from the interatrial septum, 2 from the posterocaudal wall, 2 from the root of the anterior leaflet of the mitral valve, and 1 from the left atrial roof. One left atrial myxoma was combined with severe mitral valve regurgitation. One patient was diagnosed with left atrial myxoma and patent foramen ovale (Table 6.1).
Table 6.1
The clinical data of robotic atrial myxoma resection
Variables | |
|---|---|
Total number of patients | 45 |
Gender | |
Male (%) | 12 (32.5 %) |
Female (%) | 33 (67.5 %) |
Age (year) | 47.0 ± 14.1 |
Weight (kg) | 62.3 ± 11.8 |
Height (cm) | 162.0 ± 6.8
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|