Fig. 7.1
The patient position
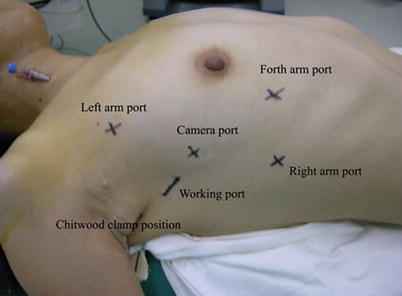
Fig. 7.2
The positions of ports
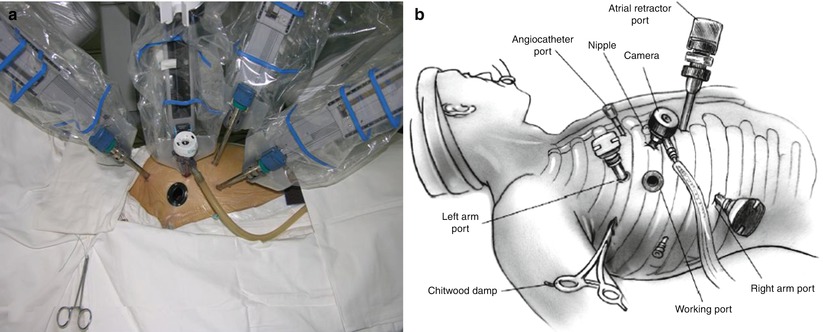
Fig. 7.3
The robotic mitral valve repair operative field. (a) System set-up; (b) Sketch map. From Siwek and Reynolds (21Reproduced with permission from Siwek and Reynolds [21)
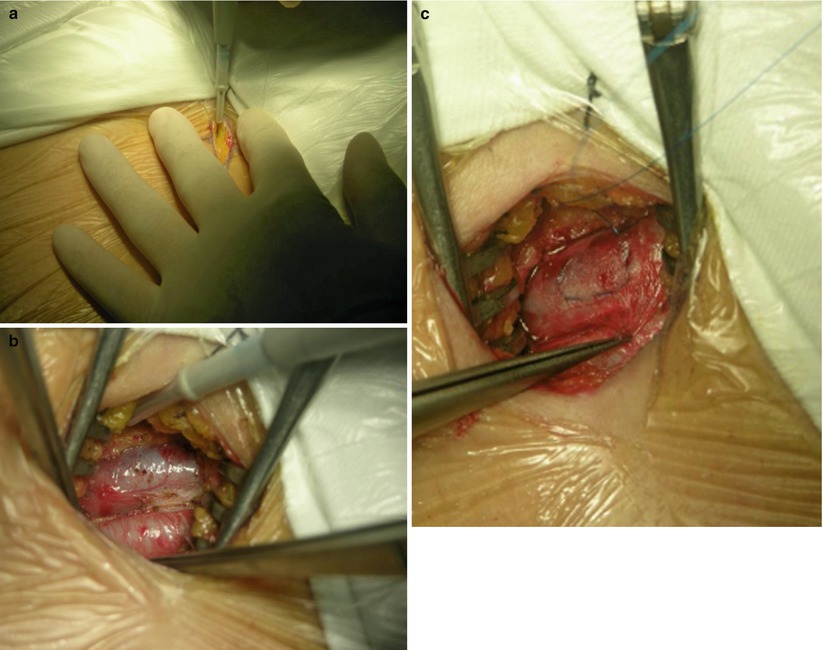
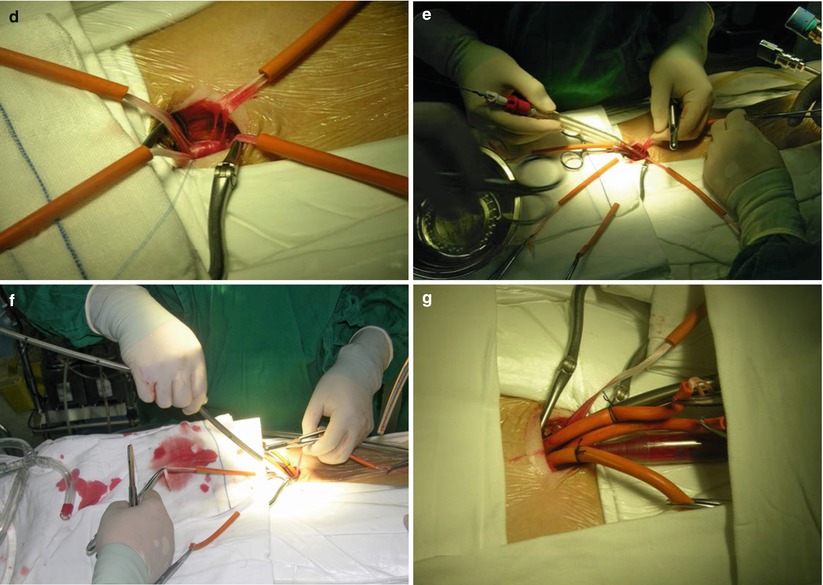
Fig. 7.4
Cannulation of the femoral artery and vein. (a) Making a 2-cm incision in the right groin crease; (b) Exposure of the femoral artery and vein; (c) Placing purse string suture on the vein; (d) Circling the vessels; (e) Arterial cannulas inserted over a guidewire; (f) Vein cannulas inserted over a guidewire; (g) Cannulation completed
7.5.3 Surgical Technique
The anterior pericardium is opened longitudinally to the phrenic nerve. The incision is extended superiorly to expose the aorta. The vertical pericardium staying sutures are placed on the right side and left superior side of pericardium for exposure, and two right side staying sutures should be as far as possible for nice exposure. The aorta is occluded with Chitwood cross-clamp via the fourth intercostal space in the midaxillary line. Care must be taken to avoid injury to the right pulmonary artery, the left atrial appendage, or the left main coronary artery. We have never used the endoballoon as an occluder since we started with robotic surgery. Antegrade cold blood cardioplegia is administered directly through anterior chest on the second or third intercostal space with 14 F angiocatheter (Fig. 7.5). Carbon dioxide is insufflated continuously into the operative field for deairing. The interatrial groove is dissected. Then a small left atriotomy is performed medial to the right superior pulmonary vein with extension toward the SVC and inferiorly behind the IVC (Fig. 7.6). The atrial septum is retracted with left atrial endowrist retractor (Fig. 7.7) to visualize the mitral valve. The mitral valve and appurtenants are inspected using a valve hook (Fig. 7.8). A left inferior pulmonary vein sump scavenges residual left atrial blood.
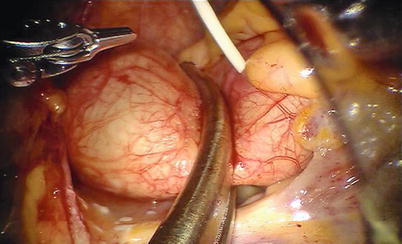
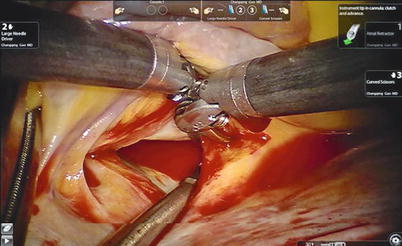
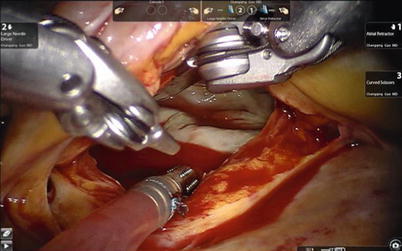
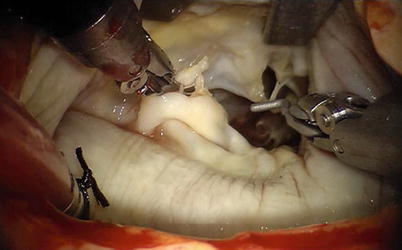

Fig. 7.5
The Chitwood cross-clamp and antegrade cardioplegia administered

Fig. 7.6
The small left atriotomy

Fig. 7.7
The left atrial retractor is used to expose the mitral valve

Fig. 7.8
The mitral valve and appurtenants are inspected using a valve hook
Valve function is tested by cold saline injections. The table surgeon exchanges the various microtipped instruments. Standard reconstructive methods have been used in all da Vinci mitral valve repairs. Posterior leaflet prolapse is treated by either quadrangular or trapezoidal resection of diseased chordal leaflet segment (Fig. 7.9). Residual leaflet edges are re-approximated with 4-0 GoreTex interrupted sutures or nitinol U-clips (Coalescent Inc., Sunnyvale, CA) (Fig. 7.10). Water injection test is performed again to identify that no mitral valve regurgitation occurs.
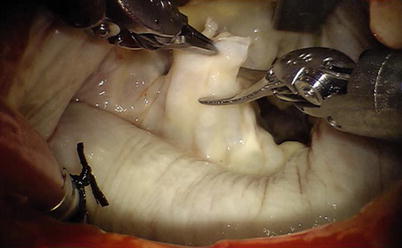
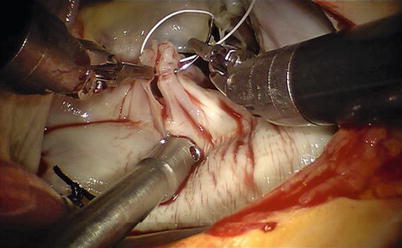

Fig. 7.9
Resecting the prolapse P2 segment

Fig. 7.10
Residual leaflet edges are reapproximated
Remodeling the annulus by ring annuloplasty after mitral valve repair is essential to a complete and long-lasting repair. The size of annulus is measured (Fig. 7.11). The flexible annuloplasty band (Cosgrove Edwards, Edwards Lifesciences, Irvine, CA) is secured between the fibrous trigones using running sutures or nitinol U-clips. Bites should be deep with the needle entering the annulus, then into the left ventricular cavity, and then coming out on the atrial side again.
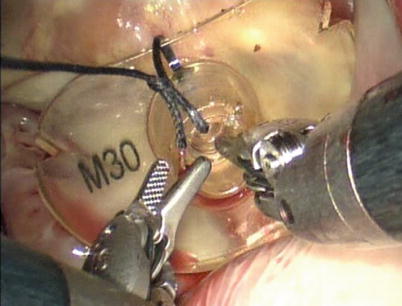

Fig. 7.11
Measuring the annulus
For the running suture technique (Fig. 7.12), three 2-0 braided polyester sutures are used to secure the annuloplasty ring as follows [20]: After the ring is introduced into the left atrium, the first suture (16 cm in length) is passed through the ring, through the right trigone, and then back through the ring. The suture is then tied down and runs clockwise to the midportion of the ring (Fig. 7.12a). The second suture (14 cm in length) is then passed through the ring, through the midportion of the annulus, and then back through the ring. This second suture is tied down, and the tail is used to secure the first suture (Fig. 7.12b). The second suture then runs clockwise to the left trigone (Fig. 7.12c). The third suture (9 cm in length) is passed through the ring, through the left trigone, and then back through the ring. This third suture is tied down, and the tail is used to secure the second suture (Fig. 7.13d). Another technique is the 2-suture method that we have used. The technique is as follows: The first suture is used as described above. The second suture is passed through the ring, through the left trigone. The suture is then tied down and runs anticlockwise to the midportion of the ring. Then, the two sutures are tied together at the midpoint of the ring (Fig. 7.13).
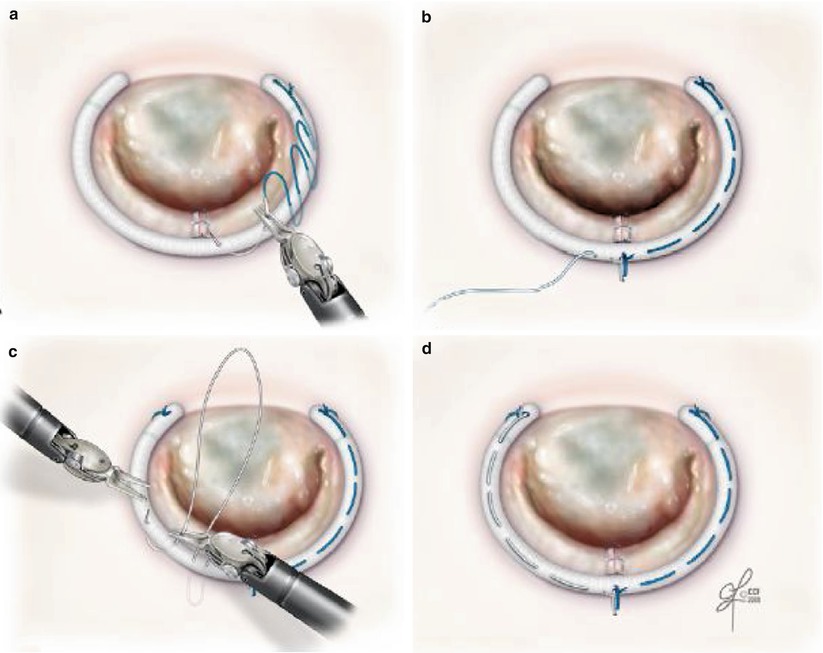
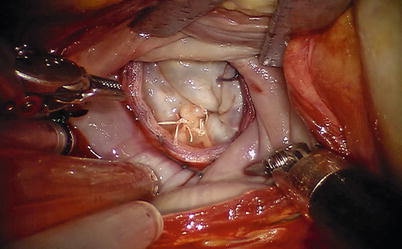

Fig. 7.12
The running annuloplasty suture technique. (a) First suture is tied at the right trigone and run clockwise. (b) Second suture is tied at the mid-portion of the annulus and the tail is tied to the first suture. (c) Second suture runs clockwise. (d) Third suture is tied at the left trigone and the tail is tied to the second suture (Reproduced with permission from Tomislav et al. [20])

Fig. 7.13
The ring is secured using the running suture technique
The flexible band can be secured using nitinol U-clips (Figs. 7.14 and 7.15). These clips are placed through the annulus like conventional sutures (Fig. 7.14a), with each arm then placed through the band. The locking mechanism, which deploys the clip, is released using robotic needle holders (Fig. 7.14b). Nitinol retains a preformed shape securing the annuloplasty band tightly against the tissues. The U-clip arms are carefully laid over the annuloplasty band to secure it firmly (Fig. 7.14c).
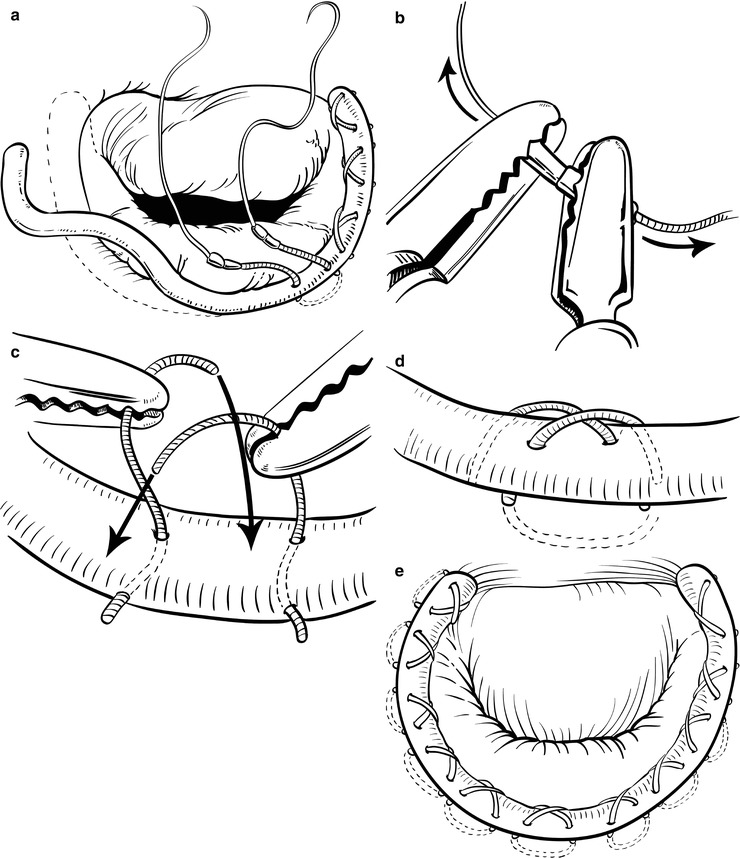
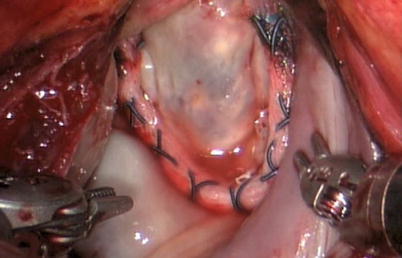

Fig. 7.14
Annuloplasty band is secured with nitinol U-clips using the da Vinci system. (a) The clips are placed through the annula. (b) The clips are released using robotic needle holders. (c) The U-clip arm is laid over the band. (d) The secured clip against the tissues. (e) The annuloplasty band is secured using U-clip

Fig. 7.15
Completed repair using nitinol U-clips to secure the annuloplasty band
The congenital clefts in anterior leaflet of the mitral valve can cause severe mitral valve insufficiency that requires surgical correction. The robotic repair can be easily used for isolated anterior cleft repair. After inspection of the intracardiac anatomy (Fig. 7.16), the cleft in the mitral valve is repaired using multiple interrupted 4-0 GoreTex suture (Fig. 7.17). The mitral valve function is tested by the cold saline injections (Fig. 7.18). In the presence of annular dilation, the repair is buttressed by an annuloplasty. The ring implanted can be completed using the method described above (Fig. 7.19).
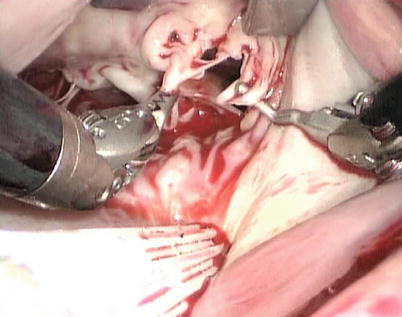
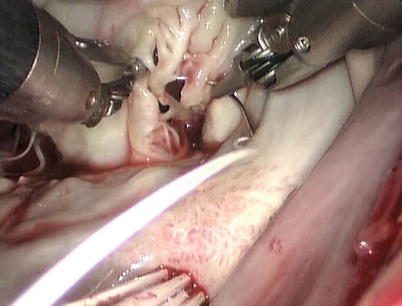
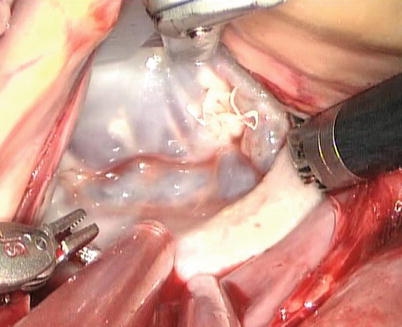
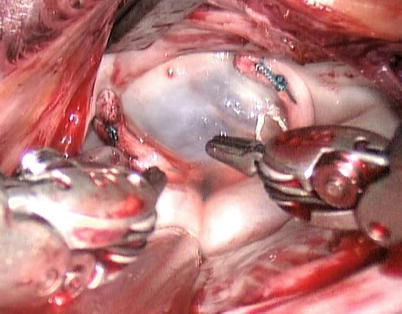

Fig. 7.16
The cleft is inspected

Fig. 7.17
The cleft is repaired using multiple interrupted 4-0 GorTex suture

Fig. 7.18
The mitral valve function I is tested by the cold saline injections

Fig. 7.19
The ring is secured using running sutures
GoreTex neo-chordae placement is greatly facilitated by a robotic approach due to excellent exposure and magnified view of the subvalvular apparatus. The minimal distortion of the valve provided by the lateral approach enhances the ability to judge and adjust chordae length. Through inspection with mitral valve apparatus, the ruptured chordae is identified (Fig. 7.20). 4-0 GoreTex buttressed with two pledgets are placed through the head of the papillary muscle (Fig. 7.21). Both ends of the suture are then brought through the leading edge of the leaflet (Fig. 7.22). The suture length is then adjusted and the suture is held with a robotic instrument while the patient side surgeon assistant ties the suture (Fig. 7.23). The surgeon firmly holds the suture and let the assistant tie against the robotic instrument to prevent the knot from slipping and making the new chordae too short [21].
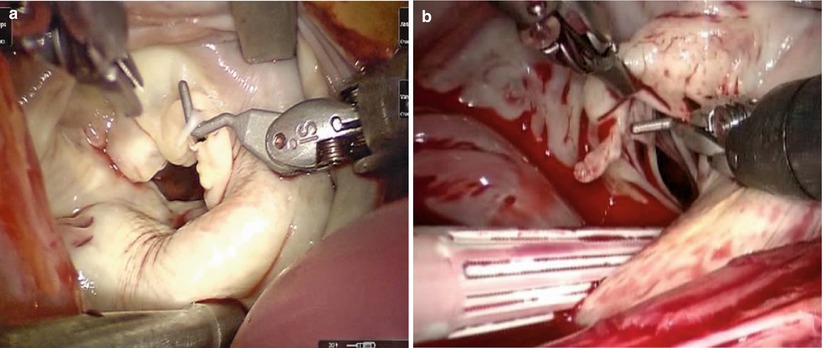
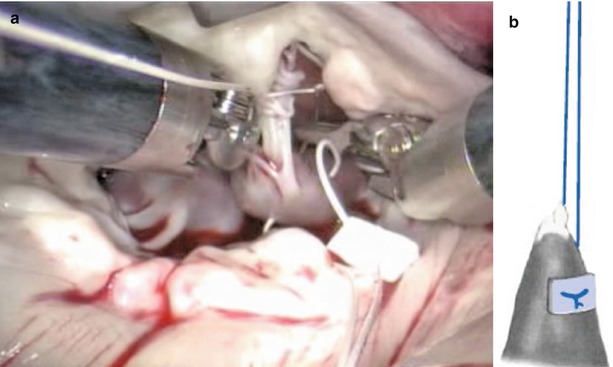
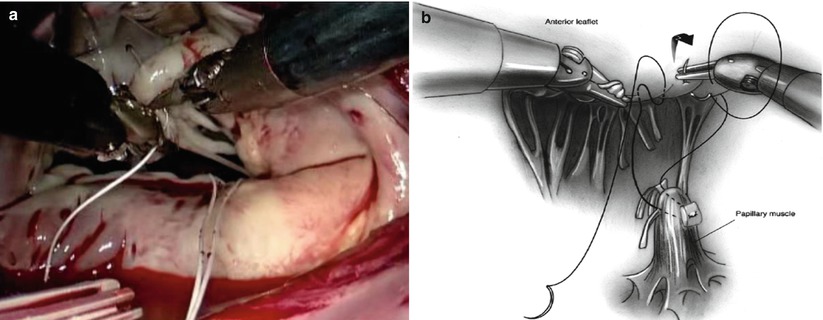
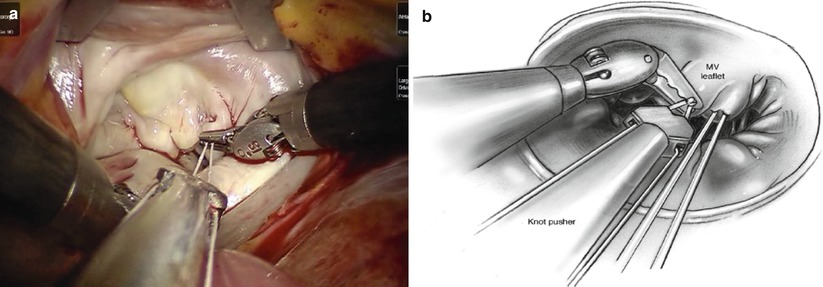

Fig. 7.20
The ruptured chordae of mitral valve. (a) Ruptured chordae in A3 area of anterior leaflet; (b) Ruptured chordae in A2 area of anterior leaflet

Fig. 7.21
4-0 GoreTex buttressed with two pledgets are placed through the head of the papillary muscle. (a) Screenshot; (b) Sketch map

Fig. 7.22
The sutures are brought through the leading edge of the leaflet. (a) Screenshot; (b) Sketch map. From Siwek and Reynolds (Reproduced with permission from Siwek and Reynolds [21])

Fig. 7.23
(a) The suture firmly held is tied against the instrument to prevent the knot from slipping and making the new chordae too short. (b) Sketch map. From Siwek and Reynolds (Reproduced with permission from Siwek and Reynolds [21])
The left atriotomy is closed with running sutures after the satisfactory mitral valve plasty using water injection test. Before the crossclamp release, the left atrium is meticulously deaired with antegrade cold blood cardioplegic solution through the angiocatheter, and after removal of the cardioplegia angiocatheter, the cardioplegia site is closed with extracorporeal knot tying through the working port, and then patient is weaned from CPB. The surgical results are confirmed by TEE (Fig. 7.24). and then chest tubes are inserted, and da Vinci was undocked finally.
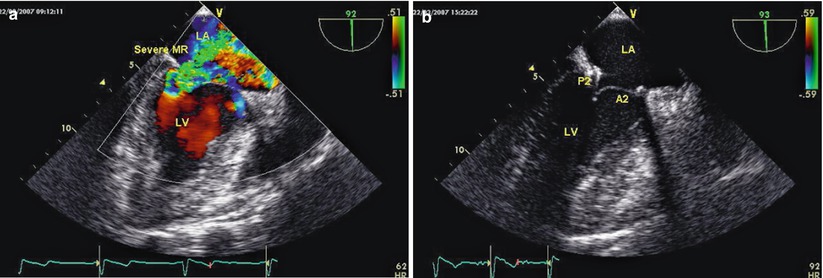

Fig. 7.24
TEE is used to evaluate the surgical result. (a) Pre-operation, severe mitral valve regurgitation; (b) Post-operative echo
7.5.4 Postoperative Management
Postoperatively patients are monitored as usual at the ICU, and discharged to an intermediate care unit as soon as their hemodynamics and spontaneous respiration have been adequately stabilized. Chest drains are removed when drainage reaches less than 50 mL/12 h. All patients undergo transthoracic echocardiography immediately before discharge from hospital and at 3 months after the operation.
7.5.5 Surgical Experience and Learning Curves
Between January 2007 and May 2013, 70 consecutive patients (49 male and 21 female) underwent mitral valve repair with da Vinci S or da Vinci Si Surgical System at PLA General Hospital. Mean age of the patients was 45.2 years (from 14 to 62 years). All the patients had isolated mitral valve regurgitation. Patients were excluded if they could not tolerate single-lung ventilation or peripheral CPB, or otherwise were considered as poor candidates for a thoracoscopic approach (Table 7.1).
Table 7.1
Preoperative echocardiographic mitral valve characteristics (n = 90)
Variables
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|