Tissue Characteristics and Contrast Agents: PD, T1, and T2
The brightness of the voxels in an MR image is directly proportional to the amount of signal measured by the receiver coils. MR pulse sequences can be designed to emphasize different tissue properties, such as proton density (PD), T1 relaxation time, T2 relaxation time, motion, vascularity, and oxygenation, among others. The basic properties of PD, T1, and T2 are reflected in all MR images to a greater or lesser extent depending on the pulse sequence design. These three properties are briefly introduced in this chapter. Most exogenous contrast agents are designed to alter the T1 or T2 relaxation times of tissues. Some of the contrast agents commonly used in cardiovascular MR imaging are reviewed.
KEY CONCEPTS
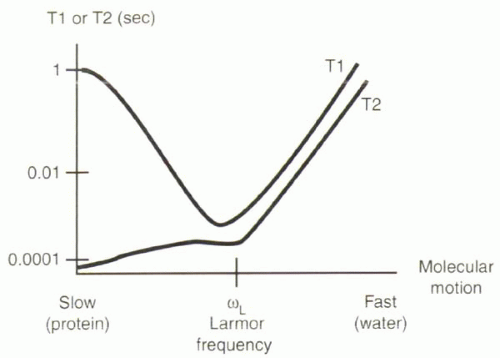
[right half black circle] T1 relaxation times reflect spin-lattice interactions. T1 times are shortest when molecular motion approaches the Larmor frequency.
[right half black circle] Gadolinium chelates are contrast agents used predominantly to shorten T1 relaxation times of surrounding protons.
[right half black circle] The relationship between the concentration of gadolinium contrast material and signal intensity is neither linear nor monotonic.
[right half black circle] T2 relaxation times reflect spin-spin interactions. T2 times are shortest for slow rates of molecular motion.
[right half black circle] Relaxivity is the inverse of the relaxation time, and relaxivity of multiple tissues or components is additive.
PROTON DENSITY
Proton density reflects the number or percentage of “MR visible” protons per unit volume. It does not refer to the number of hydrogen nuclei in a tissue. The majority of protons are generally not visible by MR because they are associated with large macromolecules and have very short T2 times (0.1 msec or less). As a percentage of total protons, MR visible protons make up about about 10% to 11% of all protons for most tissues. For example, for muscle, MR visible protons comprise 9.3% of all protons, while for cerebrospinal fluid (CSF) the value is about 10.8%. Sequences that have image contrast based on differences in proton density (called “proton density-weighted”) are useful in musculoskeletal applications to help visualize low-signal structures such as bone and ligaments, but they are not commonly used in cardiovascular MR applications.
T1 RELAXATION: SPIN-LATTICE OR LONGITUDINAL RELAXATION
The goal of this and the following section is to give the reader an intuitive understanding about why some tissues have long T1 and T2 relaxation times and others have short relaxation times. To gain this insight, it is important to appreciate differences in the molecular motion of protons in different tissues, because the local field fluctuations associated with molecular motion can promote or delay T1 and T2 relaxation.
The T1 relaxation time depends on the net transfer of energy to (or absorption from) the environment, or “lattice.” This transfer occurs only when a proton encounters a magnetic field fluctuating close to its Larmor frequency, which in turn depends on the magnetic field, B0, to which it is exposed (Figure I3-1). The field fluctuations depend on rates of molecular motion.
All molecules are in a constant state of random motion. Rates of molecular motion vary for different tissues. Water molecules in pure water are relatively small and move very quickly, so the protons in them experience field fluctuations at a frequency much higher than the Larmor frequency at 1.5 T. Proteins and other macromolecules are larger and
relatively slow-moving, so protons in them experience a field that changes much more slowly than the Larmor frequency at 1.5 T. It turns out that protons in fat have a natural frequency of motion that is close to the Larmor frequency at 1.5 T, and therefore the longitudinal relaxation of fat is fast, and its T1 time is very short. Water molecules that interact with proteins and macromolecules are slowed by their attraction to them, so protein-containing fluids also have short T1 times. Protons in the majority of tissues other than free water are at the slower end of the spectrum, slower than the Larmor frequency.
relatively slow-moving, so protons in them experience a field that changes much more slowly than the Larmor frequency at 1.5 T. It turns out that protons in fat have a natural frequency of motion that is close to the Larmor frequency at 1.5 T, and therefore the longitudinal relaxation of fat is fast, and its T1 time is very short. Water molecules that interact with proteins and macromolecules are slowed by their attraction to them, so protein-containing fluids also have short T1 times. Protons in the majority of tissues other than free water are at the slower end of the spectrum, slower than the Larmor frequency.
IMPORTANT CONCEPT:
T1 relaxation is fastest when the frequency of molecular motion is close to the Larmor frequency.
CHALLENGE QUESTION: Do tissues with short T1 times tend to be higher or lower in signal intensity on MR images compared with tissues with long T1 times?
Short T1 times mean faster recovery of longitudinal magnetization and therefore more measurable signal following each RF excitation pulse (Figure I3-2).
T1 Contrast Agents: Gadolinium Chelates
Although the intrinsic T1, T2, and proton-density differences of tissues can be a useful basis for diagnosing disease with MRI, frequently exogenous contrast agents are administered to improve the detection and characterization of pathology. In cardiovascular MRI, intravenous contrast agents are widely used for a number of reasons, including imaging vascular anatomy and pathology, assessing blood supply to normal and pathologic tissues, and evaluating myocardial viability. Contrast agents that shorten T1 relaxation times are desirable because they result in increased signal on T1-weighted images. The most commonly used agents are chelates of gadolinium (Gd3+), a heavy metal ion that alters the T1 relaxation of adjacent protons as a result of its strong paramagnetic properties. Manganese chelates (Mn2+) are less commonly used.
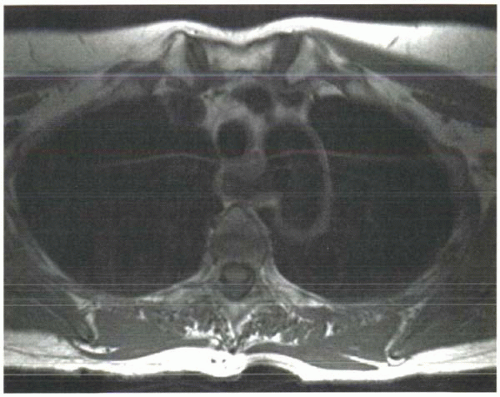 FIGURE I3-2. Effects of differences in T1 relaxation times on image signal intensity in a subject with aberrant right subclavian artery. Tissues such as fat and bone marrow have short T1 relaxation times and therefore have greater signal than tissues such as muscle. The technique used to acquire this image caused signal loss in flowing blood, hence the absence of signal in all vessels. |
Gadolinium is a lanthanide element in the family of rare earth metals. It has seven unpaired electrons. Each unpaired electron contributes to a strong magnetic moment, and therefore the gadolinium atom is paramagnetic; that is, it adds to the external magnetic field. The magnetic moment (μ) of each unpaired electron is 657 times as great as the magnetic moment of a proton. Because the rate of longitudinal relaxation is proportional to the square of the moment (μ2), gadolinium contrast agents cause nearby protons to relax a million times faster than usual. Note that the gadolinium is not directly imaged. Rather, its effect on longitudinal relaxation of protons is responsible for the measured signal change.
IMPORTANT CONCEPT:
Gadolinium chelates are effective contrast agents because they cause nearby protons to relax much faster than usual.
The Gd3+ ion has nine sites to which water molecules can bind, but for that very reason the free gadolinium ion, like other heavy metals, is toxic, because those binding sites may attach to chemical groups on the body’s proteins that normally bind to other ions such as calcium. Therefore, chelating agents are used to bind most of the sites, reducing the toxicity of the agent. The nature of the chelate determines the biodistribution and clearance of the gadolinium contrast agent. Several different gadolinium chelates are available commercially (Figure I3-3). One of the most widely used agents, gadopentetate dimeglumine, uses a diethylene-triamine pentaacetic acid (DTPA) chelate to bind eight of the nine binding sites, leaving the ninth site for water to approach the paramagnetic center of the molecule.
Properties of several commonly used commercially available gadolinium chelates are described in the following list, and their chemical structures are shown in Figure I3-3.
(1) Gadopentetate dimeglumine (Gd DTPA) [Magnevist, Berlex/Schering]
0.5 mol/L concentration
Linear structure
Ionic agent: Gd-DTPA dissociates into gadopentetate2− and 2 meglumine1+
Osmolarity: 1960 mOsm/kg (each molecule dissociates into 3 ions)
15 mL = 35 mOsm (recall that normal osmolarity of serum is 285 mOsm/L)
Dose: 0.1 mmol/kg body weight
In Europe, doses up to 0.3 mmol/kg have been approved
Biological elimination half-life: 1.6 hr (91% excreted by 24 hr)
Excretion: renal (glomerular filtration)
T1 relaxivity (r1): ˜4-5 L/mmol-s
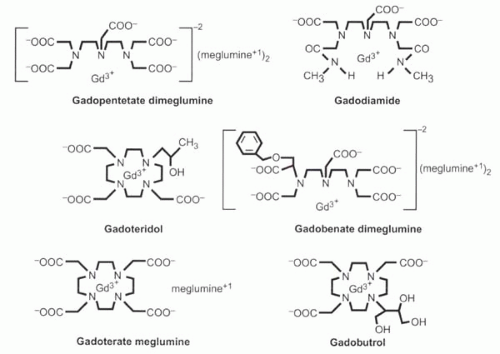 FIGURE I3-3. The most commonly used commercially available gadolinium contrast agents. |
(2) Gadodiamide (Gd DTPA-BMA) [Omniscan, GE Healthcare]
0.5 mol/L concentration
Linear structure
Nonionic agent
Osmolarity: 789 mOsm/kg
Dose: 0.1-0.3 mmol/kg body weight
Biological elimination half-life: 1.2 hr (95-98% excreted by 24 hr)
Excretion: renal (glomerular filtration)
T1 relaxivity (r1): ˜4-5 L/mmol-s
(3) Gadoteridol (Gd HP-DO3A) [ProHance, Bracco]
0.5 mol/L concentration
Macrocyclic (ring) ligand
Nonionic agent
Osmolarity: 630 mOsm/kg
Dose: 0.1-0.3 mmol/kg body weight
Biological elimination half-life: 1.6 hr (94% excreted by 24 hr)
Excretion: renal (glomerular filtration)
T1 relaxivity (r1): ˜4-5 L/mmol-s
(4) Gadobenate dimeglumine (Gd-BOPTA) [MultiHance, Bracco]
(5) Gadoterate meglumine (Gd-DOTA) [Dotarem, Guerbet] (not available in the United States)
0.5 mol/L concentration
Macrocyclic (ring) ligand
Ionic agent
Osmolarity: 1400 mOsm/kg
Dose: 0.1-0.3 mmol/kg body weight
Excretion: renal (glomerular filtration)
T1 relaxivity (rl): ˜4-5 L/mmol-s
(6) Gadobutrol [Gadovist, Schering] (not available in the United States)
1 mol/L concentration
Macrocyclic (ring) ligand
Nonionic agent
Osmolarity: 1603 mOsm/kg
Dose: 0.1-0.3 mmol/kg body weight
Excretion: renal (glomerular filtration)
T1 relaxivity (rl): ˜4-5 L/mmol-s
Dose of Gadolinium Chelates
Doses of gadolinium contrast agents are scaled to subject weight. Most of the gadolinium chelates are administered at doses of 0.1-0.3 mmol/kg body weight. The term single dose refers to 0.1 mmol/kg; a double dose means 0.2 mmol/kg. For commercial preparations that are 0.5 mol/L, a single dose corresponds to about 12-15 mL for an average-sized (60-75 kg) individual.
In terms of subject body weight in kilograms (kg) or pounds (lb), the dose calculation can be estimated as
0.1 mmol/kg = 0.2 mL/kg subject body weight
0.1 mmol/kg ≈ 0.1 mL/lb subject body weight
A table of doses for commercial preparations that are 0.5 mol/L is provided (Table I3-1).
CHALLENGE QUESTION: How many mL of gadolinium contrast material would be required to administer a single dose to a 75 kg person? What about a 100 kg person?
View Answer
Answer: A single dose equals 0.1 mmol/kg or 0.2 mL/kg. Thus, a single dose equals 15 mL for a 75 kg person and 20 mL for a 100 kg person.
Safety of Gadolinium Chelates
One measure of the safety of gadolinium chelates is their stability in vivo, which in large part depends on the how tightly the gadolinium ion is bound to its chelate. On this basis, gadoteridol (Prohance) is the most stable, while gadodiamide (Omniscan) is the least.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree