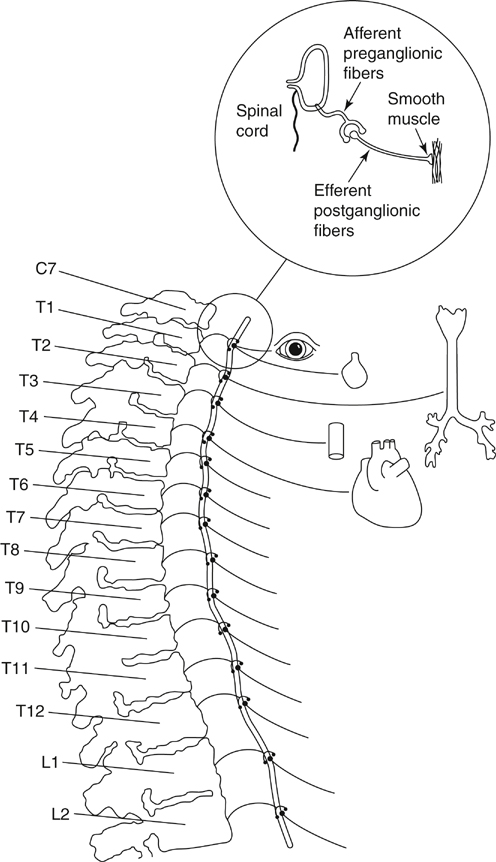
Thoracic sympathetic ganglia are the most laterally placed structures in the posterior mediastinum, and each sympathetic trunk alongside the thoracic vertebrae enters the thorax by passing anterior to the neck of the corresponding rib and exits the chest by passing behind the medial arcuate ligament of the diaphragm. In the upper part of the thorax it lies on the necks of the ribs, and in its lower course, it lies on the vertebral bodies as the vertebrae become broader. The thoracic sympathetic trunk usually contains 11 or 12 ganglia. The first thoracic and inferior cervical ganglia might fuse together in front of the neck of the first rib to form the stellate ganglion (Figure 1). Each thoracic sympathetic ganglion is connected with the corresponding spinal nerve by white and gray ramus communications. The white ramus brings preganglionic fibers from the spinal nerve to the sympathetic ganglion, and the grey ramus carries postganglionic fibers from the ganglion to the spinal nerve (see Figure 1). The preganglionic fibers from the T1 segment go mostly to the head and neck region; however, in about 10% of the population, T1 fibers supply the upper extremity as well. The 2nd, 3rd, and 4th ganglia give branches to the cardiac and pulmonary plexuses. The upper five ganglia give fine branches to the aorta to form an aortic plexus (see Figure 1). All sympathetic preganglionic fibers exit the spinal cord in the anterior nerve roots of the thoracic and first two lumbar nerves. These fibers (white ramus) either synapse in the thoracic and lumbar ganglia or run up or down through the sympathetic chain to synapse with other ganglia (see Figure 1). Some of these fibers might pass through ganglia to synapse in a more peripheral ganglion. The term white ramus is used because all of the preganglionic fibers are myelinated. The fibers leaving the ganglia are called efferent postganglionic cells; these are unmyelinated and gray.
Thoracic Sympathectomy
Anatomy of the Cervical and Thoracic Sympathetic Ganglia
Physiology of Sympathetic Nervous System
Thoracic Sympathectomy



