The femoropopliteal (FP) artery is the term used to describe the composite of the superficial femoral (SFA) and popliteal (PA) arteries. In an average adult, this artery measures approximately 50 cm in length. Atherosclerosis of the FP artery is characterized by diffuse involvement, a high incidence of progression to occlusion, a propensity for superimposed calcification, and a large plaque burden (1,2). In addition, the FP artery is flanked by two major flexion points at the hip, proximally, and knee joint, distally and courses through the muscular portion of the thigh (i.e., adductor canal). As a result, the FP artery is subject to significant torsion, bending, and both axial and longitudinal compression forces that produce significant conformational change in the vessel. These factors present a significant challenge for endovascular therapies in this artery.
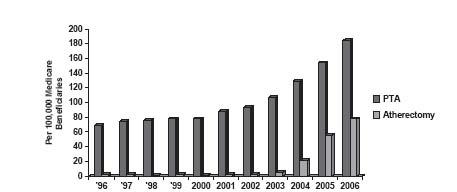
Despite these challenges, there has been a dramatic increase in percutaneous revascularization procedures in the FP artery. In the period from 1996 to 2006, there has been a rise in FP interventions from 69 to 184 per 100,000 Medicare beneficiaries (Fig. 16.1) (3). This represents nearly 55% of all lower extremity endovascular interventions, demonstrating the frequency of involvement of this artery in patients with peripheral artery disease (PAD). The major driving force behind the increased numbers of FP intervention is the increasing array of interventional tools that allow safe and successful treatment of increasingly complex disease anatomies. It is interesting that the rise in the overall number of FP procedures is paralleled by the increased use of atherectomy (i.e., a term used to describe a group of technologies that allow either ablation or removal of plaque (see Chapter 8). However, continued development of newer generations of bare-metal and covered self-expandingstents together with refinements in technique have also made a significant contribution toward the growth in FP interventional volume.
This chapter provides an overview of the approach to the interventional management of FP disease, including a description of the most commonly used technologies, and a summary of the outcomes following FP intervention.
ANATOMIC CONSIDERATIONS
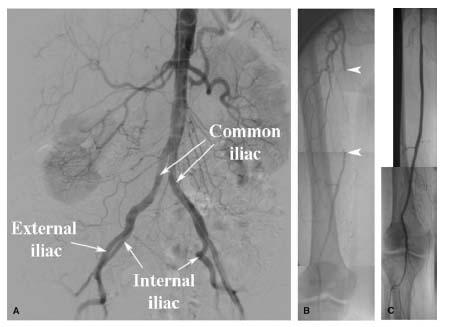
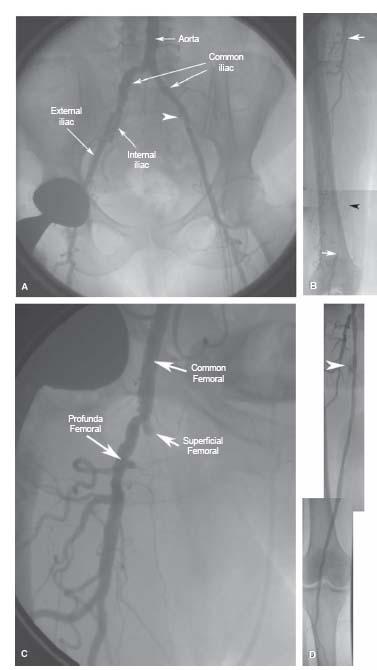
The SFA represents the direct continuation of the common femoral artery (CFA) following the origin of the profunda femoral branch (PFA) in the femoral triangle (Figs. 16.2 and 16.3). This transition from CFA to SFA usually occurs at the level of the inferior margin of the femoral head. At its origin, the SFA lies medial, and anterior, to the PFA. Proximally, it courses through the femoral triangle to reach the adductor canal. In the adductor canal, the SFA is surrounded by the muscles of the thigh: the adductor longus and magnus muscles, posteriorly, the sartorius muscle, anteriorly, and the vastus medialis muscle, medially. The SFA exits the adductor canal through the tendinous opening in the adductor magnus muscle (i.e., adductor hiatus) to reach the popliteal fossa, located in the distal portion of the posterior surface of the femur. At this point, the SFA changes its name to the popliteal artery. The popliteal artery initially runs in the popliteal fossa posterior to the distal third of the femur (Fig. 16.3), and subsequently along the posterior surface of the tibial plateau. It typically terminates by bifurcating into the anterior tibial artery and tibioperoneal trunk.
One of the distinguishing features of the SFA is the absence of significant branches throughout its course. This explains the constant diameter of this vessel, typically about 5 to 7 mm. A number of small, muscular branches may be seen, and prominent collateral branches are seen in the presence of stenotic or occlusive disease of the SFA. The only named branch of the SFA is the descending genicular branch, which usually arises in the region of the adductor canal, and contributes to the collateral circulation at the knee.
The popliteal artery provides articular branches to the capsule and ligaments of the knee joint, and genicular/sural branches that supply the calf muscles. These genicular branches are important sources of collaterals to the tibial vessels in the presence of significant PA and/or tibial artery disease.
A brief description of the anatomy of the PFA is also pertinent in a discussion of FP intervention, since it makes an important contribution to collateral flow in the presence of FP disease, and also impacts decision-making during intervention to the ostium of the SFA. The PFA arises from the lateral aspect of the CFA and runs posteriorly, and laterally, to the SFA. It gives off two major branches, proximally, the medial and lateral-circumflex femoral branches. One or both of these branches may occasionally (i.e., about 15% to 20%) arise directly from the CFA. In its mid portion and distal portion, the PFA typically gives off three or four perforating branches to the muscles of the thigh. Proximally, the medial and lateral-circumflex branches and the first perforating branch have connections with branches of the internal iliac artery (i.e., superior and inferior gluteal, and obturator branches). Distally, the lateral circumflex artery and the perforating branches have important connections with the collateral network at the knee joint, which connect with the popliteal and tibial vessels. Through these proximal and distal connections, the PFA provides an important source of collateral flow to the leg and foot in patients with significant FP disease.
Figure 16.1 • Trends in frequency of femoropopliteal artery intervention among Medicare beneficiaries between 1996 and 2006. (Modified from Goodney PP, Beck AW, Nagle J, et al. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vase Surg. 2009;50:54–60).
NONINVASIVE EVALUATION
All details regarding the noninvasive evaluation of patients with lower extremity ischemia are outlined in Chapters 2, 3, and 4.
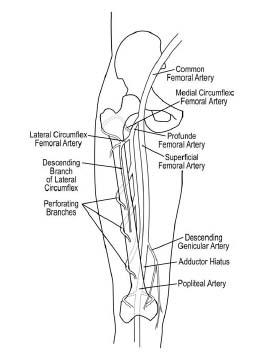
Figure 16.2 • Schematic illustration of anatomy of femoropopliteal artery (right leg).
CLASSIFICATION OF FEMOROPOPLITEAL ARTERY ATHEROSCLEROTIC DISEASE
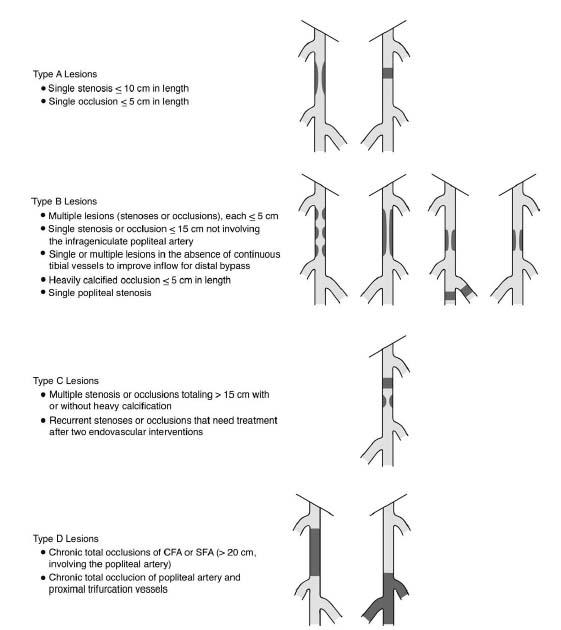
The TransAtlantic Inter-Society Consensus (TASC II) has classified FP disease into four types (i.e., A to D) based on lesion length, the number of lesions, and the presence of stenosis or occlusion (Fig. 16.4) (4).
This classification has been developed in an attempt to reach consensus regarding the optimal approaches to the revas-cularization of various lesions, and is useful when performing and comparing investigational studies of FP intervention.
INDICATIONS
Revascularization of the FP artery has traditionally been reserved for only a subset of patients with symptomatic disease, including those with severe lifestyle-limiting claudication, ischemic resting pain, or ischemic tissue loss (4). These indications reflect the approach adopted by vascular surgeons who appropriately balanced the high risk of surgical revascularization in a population with significant comorbidities, with the potential benefit to the patient. For example, the operative mortality rate for femorop-opliteal bypass ranged from 1.3% to 6%, with a perioperative risk of myocardial infarction of 1.9% to 3.4%, and a wound complication rate of 10% to 30%. The conservative approach to surgical revascularization was also supported by the recognition that 75% of patients with symptomatic peripheral vascular disease experience stabilization or improvement of their symptoms, and that conservative measures such as exercise therapy, pharmacologic therapy, and risk factor modification may provide significant improvements in walking distances and symptoms.
With the advent of endovascular techniques, the safety of FP artery revascularization even in high-risk patients was dramatically transformed, with a major complication rate with the endovascular approach of less than 1%. This has led to a major shift toward percutaneous revascularization as the therapy of choice for the indications outlined above. The superior safety of the endovascular approach has overridden the continued controversy regarding the long-term efficacy of endovascular versus surgical revascularization.
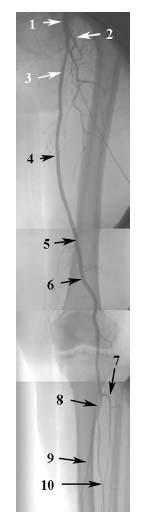
Figure 16.3 • Angiogram of left lower extremity. (1) Common femoral artery. (2) Profunda femoral artery. (3) Superficial femoral artery in femoral triangle. (4) Superficial femoral artery in adductor canal. (5) Adductor hiatus. (6) Popliteal artery. (7) Anterior tibial artery. (8) Tibioperoneal trunk. (9) Posterior tibial artery. (10) Peroneal artery.
Understandably, the shift in the risk-versus-benefit ratio provided by percutaneous revascularization of the FP artery has resulted in a broader population of patients, with less severe symptomatic disease, being treated. This practice is supported by nonrandomized data from this population showing that revascularization is associated with improved functional capacity, leg symptoms, and quality of life compared with medical therapy (5). However, randomized data would provide more reassuring evidence of the appropriateness of the broader application of FP intervention in patients with less severely symptomatic disease.
FEMOROPOPLITEAL ARTERY INTERVENTION
Diagnostic Angiography
Quality angiography of the lower extremity is an important component of the evaluation of patients with suspected FP disease. Assessment of the inflow and outflow from the FP artery determines the suitability of the patient for FP intervention and the most appropriate interventional strategy. With modern noninvasive angiographic techniques (i.e., Duplex ultrasound, computed tomography [CT], or magnetic resonance [MR] angiography), invasive angiography is no longer required to make this assessment.
If these noninvasive techniques are not available, or are suboptimal, invasive angiography remains essential. For diagnostic studies, the most common access site chosen is the CFA contralateral to the leg with the worst symptoms (i.e., assuming symptoms are bilateral and asymmetric), since this allows for a subsequent attempt at endovascular revascularization at the same sitting if an intervention is required. A pigtail catheter is advanced over a wire and placed in the distal abdominal aorta, and a static pelvic angiogram (in ipsilateral and contralateral oblique views) spanning the aortic bifurcation to the CFA is performed (i.e., power injector settings: 15 mL/sec for a total of 30 mL). If the CFA bifurcation is not clearly visualized, ipsilateral oblique views are usually helpful in displaying the origins of the SFA and PFA.
At this point, visualization of the infrainguinal arteries may be performed using one of the two methods: (1) sequential static overlapping digital subtraction angiography (DSA) at multiple levels. The authors prefer this method and perform it by placing a multiple side-holed diagnostic catheter (e.g., straight flush) in the ipsilateral (i.e., lower extremity of interest) external iliac artery (EIA) or CFA from the contralateral CFA access. Ipsilateral oblique imaging of the lower extremity is then performed (3 to 4 cc/sec for total of 9 to 12 cc/sec). If the other limb needs to be imaged, selective angiography through the side arm of the CFA sheath is performed using the same method; and (2) the bolus-chase technique. With this technique, a contrast bolus is injected and continually imaged as it progresses distally. Both limbs may be visualized with a pigtail catheter placed in the distal abdominal aorta (i.e., power injector settings: 15 mL/sec for a total of 90 mL), or an individual limb may be visualized with a multiple side-holed, straight flush catheter in the ipsilateral EIA or CFA (i.e., power injector settings: 15 mL/sec for a total of 45 mL). A 15″ image intensifier (minimum) is required for visualization of both lower extremities. With smaller image intensifiers, each leg must be visualized separately.
With modern imaging systems, the need for a central wedge placed between the legs and lateral wedges placed lateral to each leg is rare. Placing either a rigid radiopaque ruler or flexible radiopaquetape in the field of view during diagnostic angiography of the lower extremities provides a useful landmark for the analysis of lesion lengths and positioning of equipment during endovascular procedures and is strongly recommended.
Figure 16.4 • TASC II classification of femoropopliteal artery disease. (Adapted from Norgren L, Hiatt WR, Dormandy JA, et al. on behalf of the TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vase Surg 2007;45:S5-S67.)
The adjunctive pharmacologic therapies administered during FP artery intervention are similar to those used in most other peripheral vascular procedures. In clinical practice, patients receive aspirin preprocedurally. Unfractionated heparin is the anticoagulant of choice, with a target activated clotting time (ACT) of approximately 250 seconds. In complex FP intervention involving the treatment of long occlusive disease, the authors often aim for an ACT of 250 to 300 seconds. Low molecular-weight heparins or direct thrombin-inhibitors may be used in specific circumstances, but there are no data to support their routine use. Most operators reserve glycoprotein (GP) IIb/IIIa inhibitors for cases complicated by thrombosis.
Access for Intervention
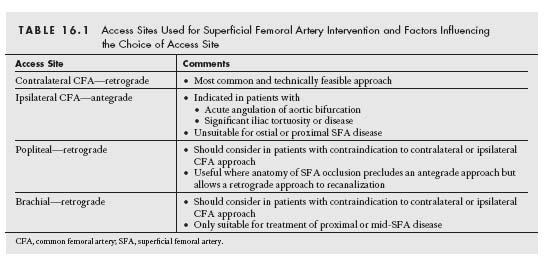
The success and ease with which any endovascular procedure may be performed rely on the platform constructed to perform the procedure (Fig. 16.5). This begins with the choice of arterial access (Table 16.1).
CONTRALATERAL CFA RETROGRADE ACCESS
When approaching an FP lesion, the most commonly chosen and technically simple access is the contralateral CFA, approached in a retrograde manner (Fig. 16.6). The modified Seldinger technique is used to gain access in the contralateral CFA.
A catheter (e.g., IM, Sos, Simmons) is then advanced over a wire into the distal aorta and used to selectively engage the ostium of the contralateral common iliac artery. A stiff-angled glidewire is then advanced with care into the CFA, and the diagnostic catheter is then advanced over the wire into the CFA. The glidewire is then exchanged for a supportive guidewire (e.g., SupraCore, Superstiff Amplatz wire with 1 cm soft tip). The catheter and short femoral sheath are then removed over the stiff wire, and a long kink-resistant sheath approximately 40 to 50 cm in length (e.g., Balkin Contralateral, Pinnacle Destination, Ansel, Raabe) is advanced into the contralateral CFA. The sheath should always be advanced in combination with the dilator, to avoid trauma to the iliac vessels.
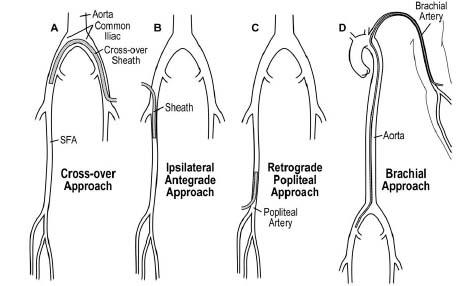
Figure 16.5 • Illustration of array of access-site strategies for femoropopliteal artery intervention.
IPSILATERAL CFA ANTEGRADE ACCESS
An antegrade, ipsilateral CFA access may be desirable in patients with specific anatomic issues involving the iliac arteries, including: severe tortuosity or disease in either iliac, or acute angulation of the native or reconstructed (i.e., surgical [e.g., aortobifemoral bypass]) or percutaneous (e.g., kissing stenting) aortoiliac bifurcation (Fig. 16.7).
Although antegrade access provides improved back-up support, compared with the contralateral CFA approach, it is uncommon for this to be a significant issue during FP intervention. In patients with ostial or proximal SFA disease, the antegrade approach may be contraindicated, owing to insufficient room in the CFA to allow a sufficient length of sheath for stable sheath placement (Fig. 16.8).
Particular caution should be exercised in using the ante-grade CFA approach in obese patients, and this approach is relatively contraindicated in the morbidly obese, owing to the increased risk of bleeding.
The technical aspects of obtaining antegrade CFA access are outlined in Chapter 6.
IPSILATERAL POPLITEAL RETROGRADE ACCESS
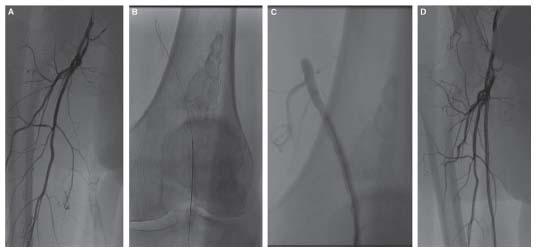
The ipsilateral popliteal retrograde approach is rarely used for FP artery intervention. However, in situations in which both the contralateral CFA and ipsilateral CFA approaches are contraindicated (e.g., severe iliac tortuosity and ostial/proximal SFA disease), it may be required. A further indication for the popliteal approach is the presence of an FP occlusion that may not be crossed using the antegrade approach. This may be due to a flush occlusion of the SFA (Fig. 16.9) or an occlusion more distally in the FP artery that cannot be crossed using an antegrade approach due to the presence of a bridging collateral or branch vessel adjacent to the proximal stump (Fig. 16.10). If there is a definable stump, distally, that is sufficiently proximal to the popliteal artery to allow safe placement of a sheath, then a retrograde popliteal approach is indicated.
The technical aspects of PA access are outlined in Chapter 6. Typically, the FP lesion is crossed using the retrograde approach, but recanalization and delivery of equipment occur via the antegrade approach since this minimizes the sheath size in the PA.
BRACHIAL ACCESS
Brachial artery access is not a particularly useful access site for FP intervention. Current limitations in the length of the delivery systems of interventional equipment other than balloons limit the type of FP disease that may be approached using this access (e.g., treatment of instent restenosis). Stents, atherec-tomy devices (with exception of laser), and re-entry tools have a maximum length of 135 cm, which limits the ability to use these devices beyond the level of the proximal SFA from the brachial approach.
RETROGRADE TIBIAL/PEDAL ACCESS
In patients with an occlusion of the popliteal artery that extends to involve the origin of the tibial vessels, retrograde access in either the posterior tibial or dorsalis pedis artery (rarely the peroneal) may be required to cross the occlusion in retrograde fashion, followed by antegrade recanalization. The details of retrograde tibial/pedal access are outlined in Chapter 6.
Interventional Technique
Before proceeding with an intervention in the FP artery, one should be certain that the access sheath size chosen accommodates the interventional equipment required to treat the lesion. In general, when using the contralateral CFA approach, the authors use a 7-Fr. sheath. This will accommodate all balloons (4 to 5 Fr.), bare-metal self-expanding stents (5 to 6 Fr.), covered self-expanding stents (7 Fr.), re-entry tools (6 to 7 Fr.), and atherectomy (7 Fr.) devices that are typically used for FP intervention. When using the antegrade approach, every effort should be made to minimize the sheath size because of the risk of access-site bleeding; the authors most commonly use a 6-Fr. sheath, but will use a 7-Fr. sheath if the use of an atherectomy device or covered stent is anticipated.
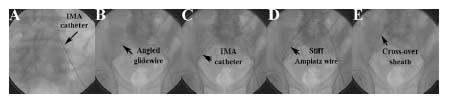
Figure 16.6 • Technique used to deliver cross-over sheath from left common femoral artery (CFA) to right CFA. A: Internal mammary (IMA) catheter at origin of right common iliac artery. B: Angled glidewire advanced into right CFA. C: IMA catheter advanced over wire to right CFA. D: Angled glidewire exchanged for 1 cm tipped stiff Amplatz wire. Note the change in shape of the aortic bifurcation caused by the stiff Amplatz wire. E: Cross-over sheath delivered over stiff Amplatz wire.
Figure 16.7 • Influence of anatomy of iliac vessels and aortic bifurcation, and superficial femoral artery (SFA) lesion on access strategy for femoropopliteal artery intervention. A: Pelvic angiogram showing acute angulation of aortic bifurcation and significant tortuosity of the right external iliac artery. B: Angiogram of the right SFA showing occlusion (margins denoted by white arrowheads) in the midportion of the vessel. There is a sufficient length of normal vessel in the proximal SFA to allow placement of a sheath placed antegrade in the ipsilateral common femoral artery. The occlusion was crossed with a 0.035″ wire, dilated with a 5.0 × 100-mm balloon, and stented with a 7.0 × 120-mm nitinol self-expanding stent that was postdilated with a 6.0 × 60-mm balloon. C: Final angiography following angioplasty and stenting.
TREATMENT OF STENOSIS
Having obtained arterial access, the treatment of stenosis in the FP artery is generally straightforward (Fig. 16.11). A soft-tipped, 0.035″ wire (e.g., Magic Torque or Wholey wire), either alone or supported by an angled glide catheter (e.g., Glide-cath), is usually successful in crossing the lesion. Use of 0.035″ wires is reasonable where the intent is to stent the lesion, since most of the stents used in the FP artery are 0.035″ compatible. Alternatively, a stiff-bodied 0.014″ wire with a soft tip (e.g., GrandSlam, Abbott Vascular) supported by a 0.014″ or 0.018″ compatible balloon may be used. Occasionally, a 0.014″ wire with a highly steerable tip (e.g., MiracleBros 3.0, Abbott Vascular) may be required to negotiate diffuse eccentric disease. This wire should be exchanged for a soft-tipped wire once the lesion has been crossed. Since all of the atherectomy devices are only compatible with 0.014″ wires, these wires should be used when the use of such devices is anticipated. The tip of the wire should be parked in the distal PA or proximal tibial vessel to provide sufficient support to deliver interventional equipment. Care should be taken with the wire in tibial vessels, particularly in the presence of disease in that location. A more easily torqued hydrophilic wire (e.g., floppy glidewire, stiff-angled glidewire) is rarely required. It is important to exchange this wire immediately for a soft-tipped nonhydrophilic wire having successfully crossed the lesion, as its routine use is associated with an increased risk of arterial injury.
ANGIOPLASTY
The angioplasty balloon size and length are matched to the vessel size (i.e., balloon-to-artery ratio of ~ 1) and lesion length in the FP artery. In most patients, the balloon diameter will be 5 or 6 mm. Lesion length measurements should be made off the radiopaque ruler or tape in the field of view. In general, the authors choose a balloon length that covers the lesion and at least 1 to 2 cm at either end of the lesion. The inflation should be performed with the minimum pressure that releases the constriction to minimize barotrauma. If the diameter of the vessel is in question, it is prudent to choose an undersized balloon and progressively increase the diameter. Improved angiographic results may be accomplished with prolonged inflations lasting 1 or more minutes.
After angioplasty, the lesion must be carefully examined for the presence of flow-limiting dissections. Dissections are commonly seen, but are not usually flow-limiting. DSA tends to underestimate the severity of dissection, which emphasizes the importance of carefully examining the unsubtracted fluoroscopic images. Evaluating the lesion for the presence of a hemodynamically significant gradient is a crucial part of evaluating the lesion after angioplasty. Regardless of the angiographic appearance, if the lesion is not associated with a residual gradient (assessed using a 4-Fr. endhole catheter or pressure wire (e.g., FloWire, Volcano or PressureWire, St Jude—formerly RADI medical system)), consideration should be given to accepting the angioplasty alone result. For focal disease, angioplasty usually provides a reasonable acute result that is durable. However, for diffuse disease, angioplasty is often associated with a significant residual gradient across the length of the diseased segment, and long-term durability is extremely poor.
Figure 16.8 • Treatment of complicated right superficial femoral artery (SFA) occlusion. A: Pelvic angiogram showing benign aortic bifurcation and iliac vessels allowing contralateral access and cross-over technique. B: Angiogram of SFA showing long occlusion of the right SFA extending from the origin to the mid-popliteal artery (arrows). Note the previously placed stent in the distal SFA (arrowhead). The extension of the occlusion into the mid-popliteal artery makes access to the true lumen just distal to the occlusion critical. C: RAO oblique view of the common femoral artery (CFA) bifurcation clearly delineating the stump of the SFA. Access was obtained in the left CFA, and a cross-over sheath was placed in the right CFA. The occlusion was crossed with a stiff-angled glidewire dissected through the subintimal place. Access to the true lumen distally was obtained using the Pioneer catheter. Angioplasty of the occlusion was performed using a 6.0 mm × 10-mm balloon, and the entire occlusion sequentially stented using 7.0 mm diameter nitinol self-expanding stents. The stents were postdilated with the 6.0 mm diameter balloon. D: Angiogram following angioplasty and stenting shows minor perforation in the proximal SFA that was treated by prolonged balloon inflation and reversal of anticoagulation.
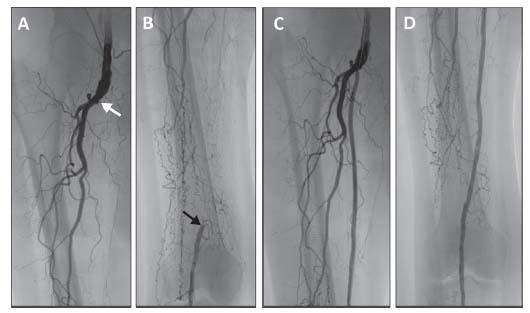
Figure 16.9 • Flush occlusion of right superficial femoral artery (A) requiring retrograde popliteal artery access (B,C) to achieve successful revascularization (D).
STENTING—BARE NITINOL SELF-EXPANDING STENTS
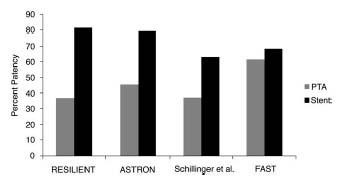
There remains considerable controversy regarding the utility of adjunctive stenting in the FP artery. The initial experience with balloon-expandable Palmaz stents and the stainless steel Wallstent was disappointing (see “FP Artery Intervention – Clinical efficacy Outcomes”). This led to the development and application of nitinol self-expanding stents in this location. There is no doubt that current generation nitinol self-expanding stents are remarkably safe and easy to use, and provide a very predictable and effective acute angiographic result compared with angioplasty, particularly for longer and more complex lesions. The major limitation of stents is the poor long-term patency associated with stenting (see “FP Artery Intervention – Clinical Efficacy Outcomes”). Despite this, four randomized trials comparing current generationnitinol self-expanding stents with angioplasty appear to demonstrate a significant benefit for stenting over angioplasty in the treatment of intermediate length lesions (5 to 10 cm) (6–9) (Fig. 16.12). Unfortunately, these studies are relatively limited in the duration of follow-up (i.e., 12 to 24 months), and there appears to be continued decline in patency rates of stents out to 4 to 5 years where patency rates may drop to 40%. Longer lesions have not been studied in a randomized controlled trial, and it is felt that stenting does not provide a significant benefit over angioplasty in shorter lesions (i.e., 5 cm). Perhaps more problematic than the high rates of restenosis associated with stenting is the lack of an effective treatment for instent restenosis. Recurrence rates of over 70%, regardless of the treatment modality, have been reported (10).
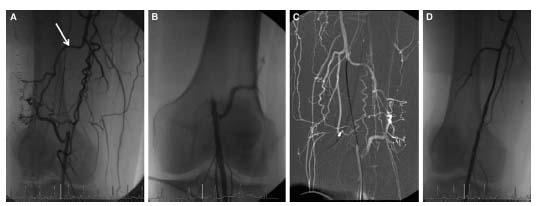
Figure 16.10 • A: Occlusion of distal superficial femoral artery with the absence of proximal stump and presence of large collateral (arrow) preventing antegrade approach to recanalization. B: Retrograde popliteal artery access was obtained. C: The lesion was crossed in a retrograde fashion with a glidewire supported by an angled glide catheter. D: Revascularization was achieved from the antegrade approach using a Silverhawk LX-M device and balloon angioplasty (5 mm × 10 cm balloon).
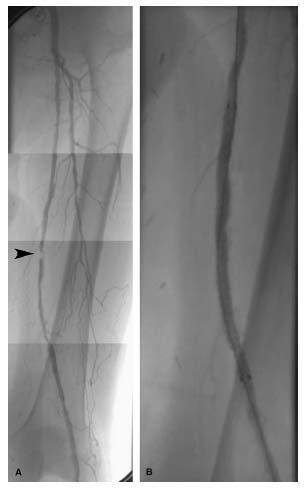
Figure 16.11 • Treatment of a superficial femoral artery Stenosis. A: Angiogram of the left superficial femoral artery shows a focal severe lesion in the mid- portion of the vessel (arrowhead), with moderate diffuse disease proximal and distal to the lesion. The focal lesion was dilated with a 4.0 × 40-mm balloon, and the treatment site stented with a 7.0 × 100-mmnitinol self-expanding stent that was postdilated with a 5.0 × 80-mm balloon. B: Final angiogram of the treatment site following intervention.
These data have important implications for the clinical indication for using stents in the treatment of FP disease. Clearly, where angioplasty or other interventional technique does not provide an adequate acute result (i.e., residual gradient, flow-limiting dissection, abrupt closure, severe residual stenosis), then stenting is appropriate. In younger patients with claudication, the implications of the high rate of restenosis and need for repeated intervention need to be discussed prior to treatment. In patients with critical limb ischemia, stenting is a reasonable strategy as stenting provides a very reliable and effective hemodynamic result in the initial 6 to 9 months, which is typically sufficient to allow complete wound healing. Since the amount of blood flow required to maintain tissue integrity is exponentially less than that required to heal a wound, restenosis in this circumstance is often asymptomatic. The exception is the patient who presents with ischemic rest pain, where restenosis is more likely to be symptomatic.
Figure 16.12 • Primary patency rates at 12 months (by Duplex ultrasound) in the four currently published trials of balloon angioplasty versus bare-metal nitinol self-expanding stents in the treatment of femoropopliteal artery disease.
There are a wide variety of bare-metal nitinol self-expanding stents currently used during FP intervention. Only two are FDA approved for this indication—the IntraCoil and Life Stent. The former is rarely used in current practice. Other self-expanding stents used in FP intervention are used off-label. All of these stents are delivered over 0.035″ wires, but may be delivered over supportive 0.014″ wires (provided the anatomy of disease permits). The most common stent diameters used in the FP location range from 5 to 7 mm. In this regard, males generally have significantly larger FP diameters compared with females. Stent diameters are chosen to be at least 1 mm larger than the reference vessel diameter. Since lesion lengths are often long, stent lengths are typically long (up to 150–200 mm). It is generally believed that using a single long stent is preferable to using overlapping stents. Although some operators perform primary stenting (i.e., no predilatation) in the FP artery, the authors routinely perform predilatation prior to stenting. Stents are postdilated with a balloon sized to the reference vessel diameter. If in doubt, the authors would recommend a conservative approach to postdilatation balloon sizing, as these stents continue to exert significant outward force over time. Many stents that acutely appear to be inadequately expanded appear perfectly cylindrical in follow-up. Achieving sufficient postdilatation to remove any pressure gradient with brisk antegrade flow should be the desired endpoint. Overag-gressive postdilatation does increase the risk for perforation, which should be avoided at all costs.
Most operators have a preference for a particular bare-metal nitinol self-expanding stent that they use in the FP artery. Having multiple different stent types available is not practical in most laboratories. However, in the authors’ opinion, having one stent that has high radial strength (e.g., SMART, Cordis) and another that is highly flexible (e.g., LifeStent, Bard) makes some sense. High radial strength is needed for the treatment of heavily calcified disease, and highly flexible stents are useful when stenting the distal SFA and popliteal artery that appears to be subject to greater physical forces than the proximal/mid-SFA.
STENTING—COVERED SELF-EXPANDING STENTS
The high rates of restenosis associated with bare-metal nitinol self-expanding stents in the FP artery have spurred interest in the use of covered self-expanding stents (see Chapter 7) in this location. In theory, the material covering these stents should prevent the ingrowth of neointima, which is the mechanism of failure for bare-metal nitinol self-expanding stents. This has been borne out in clinical practice, with the main mechanism of failure for covered stents being the development of edge restenosis at the stent margins. Since the Viabahn stent is the covered self-expanding stent with FDA approval in this location, the remaining discussion will relate to this stent only. A randomized comparison of the Viabahn stent versus femoropopliteal bypass using prosthetic conduit showing reasonable patency data at 2-year follow-up that was equivalent to the surgical group (11,12) together with a series of observational studies has lent some credibility to this strategy (13,14).
There are a number of very important practical issues when using these covered self-expanding stents in the treatment of FP disease. Case selection is of the utmost importance and largely relates to the risk of stent thrombosis. There should be adequate run-off distal to the treated segment. A minimum of straight-line flow in one tibial vessel is required. In addition, the proximal and distal margins of the stent should be placed in relatively nondiseased segments. This often results in stenting to the level of the ostium of the SFA, and the use of multiple overlapping stents. Use in FP segments of less than 4 mm diameter is not recommended, and ideally the vessel diameter should be ≥5 mm. Lesions that are noncompliant and that do not respond to predilation or other treatment strategy (e.g., atherectomy—see below) should not be treated. The result of these restrictions is that the spectrum of disease that may be treated with these stents is limited. Finally, patients who are unable to tolerate dual antiplatelet therapy (with aspirin and clopidogrel) for at least 3 months following the procedure should not be treated using these stents.
One of the major drawbacks of covered stenting in the FP artery is that important collateral channels adjacent to the diseased segment of the FP artery are occluded during stent deployment. In the proximal and mid-SFA, this is not usually of a major concern, as there is an adequate length of remaining SFA and popliteal artery to provide connection with collaterals from the profunda femoral artery in the event of stent failure. However, as one moves more distally in the SFA and particularly in the PA, this is of particular concern. If the covered stent fails, the loss of these collateral communications may increase the chance that the patient may present with a more severe degree of chronic limb ischemia than the initial clinical presentation. In addition, acute thrombosis of the covered stent may result in a clinical presentation with acute limb ischemia, although preliminary data from the recent VIBRANT trial suggested that presentation with acute limb ischemia from stent failure with the Viabahn stent is no more frequent than with stent failure using a bare-metal nitinol self-expanding stent. In summary, the decision to use a covered stent in the FP artery requires a careful assessment of the patient and disease anatomy with a thorough understanding of the implications of treatment.
From a practical standpoint, the Viabahn stent should always be delivered over a stiff-bodied 0.035″ wire (e.g., SupraCore, Stiff Amplatz wire). The choice of stent diameter is much more critical with the Viabahn stent compared with bare-metal nitinol self-expanding stents, which can be generously oversized with little consequence. The Viabahn stent diameter should never be more than 1 mm larger than the reference diameter. Oversizing of the Viabahn stent can result in the creation of folds in the stent lining, which is felt to increase the risk of stent thrombosis. Some operators use intravascular ultrasound (IVUS) to accurately measure the lumen diameter and avoid errors in this assessment. Given the need to cover from relatively normal segment proximally to normal segment distally, the stent length chosen should be adequate to meet this requirement. Following careful positioning of the stent, the stent delivery system is immobilized by having an assistant hold the system near the entrance to the access sheath and the primary operator holds the system near the hub of the stent. Deployment by pulling on the string from the side arm at the hub of the stent should be slow. If these maneuvers are implemented, the stent will remain very stable, and very accurate stent placement is possible. It is important to be aware that once the stent is seen to deploy, it cannot be repositioned. The stent does not foreshorten like bare-metal self-expanding stents, a feature that is very helpful when treating ostial SFA disease. Postdilation is recommended using a noncompliant balloon that is sized to the reference vessel diameter.
ATHERECTOMY
The range of plaque removal and plaque ablation devices used in the treatment of infrainguinal disease is described in detail in Chapter 8. Only a brief comment related to their use in the FP artery is provided here.
Few technologies have engendered such polar opinions in the peripheral space as atherectomy devices. However, there is no doubt in the authors’ minds that these devices allow for the treatment of a broader range of disease anatomies in the FP artery than is possible using conventional balloon and stent technologies and reduce the need for stenting to achieve an acceptable angiographic and hemodyamic result (15).
The specific disease subsets in which these technologies are useful in the FP artery include:
- Ostial disease of the SFA—Debulking at the SFA ostium reduces the risk of plaque shift into the PFA. Given the directionality of the Silverhawk catheter, this catheter is favored for this indication.
- Severe calcific disease—The two systems favored by the authors for this indication include the Diamond-back 360° and TurboHawk catheter. The Diamondback tends to be used for the most severe focal calcific disease, whereas the TurboHawk is used for more diffuse calcific disease.
- Diffuse disease—The Turbo Elite Laser and Jetstream® catheters have particular application in the treatment of diffuse disease due to the ability to treat a long length of disease with the single introduction of the catheter and the lower embolic risk with these catheters compared with the Diamondback 360° device. The Silverhawk catheters can be used to treat diffuse disease, but multiple introductions of the catheter can become time-consuming and lead to catheter failure.
- Popliteal artery disease. Since stenting with bare-metal nitinol self-expanding stents in this location is contraindicated, any of the plaque removal or ablation devices that can achieve a successful angiographic and hemodynamic result in this location without the need for a stent represent an important advance over angioplasty alone.
There are no randomized comparative studies of atherectomy versus conventional balloon/stent treatments in the FP artery. It is widely believed that long-term restenosis rates with these technologies are unlikely to differ significantly from angioplasty or stenting. These technologies do have complications associated with their use (see “Complications of FP artery intervention”). In particular, the risk of distal embolization needs to be acknowledged. The authors have a very low threshold for using distal embolic protection with these devices using the characteristics of the lesion (e.g., length of disease, presence of thrombus), the quality of the distal runoff, and the clinical indication for the procedure (i.e., claudication vs. critical limb ischemia) to help stratify those patients at greatest risk from embolization. In the authors’ experience, there is often evidence of embolization following the use of atherectomy devices (evidenced by filling defects in the filter) that is further exacerbated following angioplasty (± stenting), sometimes to the point of having no flow in the vessel. In such situations, the authors are careful to only partially collapse the filter, ensnaring only the proximal loop of the filter within the recovery sheath, followed by removal of the filter. The intention is to try to prevent loss of embolic material into the distal circulation during filter retrieval.
When EPDs are used, the authors prefer to use either the Emboshield® Nav6 (Abbott Vascular) or Spider (ev3) filters. The Emboshield® Nav6 filter can be used with its own Bare-Wire or with the 0.023″ Viper wire (with the 0.023″ distal tip serving as the brake for the filter). This makes it the ideal filter for use with the Diamondback 360° device. Either filter can be used with the remaining atherectomy devices.
TREATMENT OF OCCLUSIONS
Occlusions of the FP artery represent a significantly greater technical challenge compared with stenoses. These occlusions are typically very long, beginning in the proximal third of the SFA and extending to the level of the adductor hiatus and sometimes beyond into the PA (Fig. 16.13). In crossing these occlusions, there are three tasks that need to be accomplished:
1. Break through the proximal cap: In most circumstances, the proximal cap is easily breached using a straight-tipped stiff-bodied glidewire supported by a 4 or 5 Fr. angled glide catheter. If the occlusion extends beyond the distal SFA, it is important to use a 125 cm length glide catheter to facilitate wire exchanges. Our practice is to engage the proximal stump directly with the angled glide catheter, and then tap the proximal stump with the straight-tipped-stiff-bodied glidewire. Once the proximal cap is breached, the glide catheter is advanced over the glide-wire into the proximal segment of the occlusion to establish the channel for recanalization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree