Fig. 1.1
A complete developmental history of SOX9 lung epithelial progenitors. Optical projection tomography (OPT, top row, scale: 250 um) and confocal images (bottom row, scale: 20 um) of immunostained whole embryo (E9.5), lungs (E10.5–E14.5) and lobe edges (E16.5–P21). SOX9 progenitors are indicated by arrowheads. Open arrowhead: lung bud forming on the foregut. Adapted from [3]
Temporal: The respiratory primordium forms in the ventral portion of the anterior foregut around embryonic day (E) 9 in mouse and gestation week 5 in human [1, 5]. A combination of Wnt, Bmp, and Fgf signalings is required to activate the respiratory lineage factor Nkx2.1 [6–10]. The Nkx2.1 respiratory primordium gives rise to both the trachea and the lung. A subset of Nkx2.1 cells express Sox9, constituting the lung epithelial buds that will subsequently undergo branching morphogenesis (Fig. 1.1) [3].
After specification, Sox9 progenitors undergo branching morphogenesis forming new branches at an exponential rate. The basic unit of a branch consists of a branch tip and a branch stalk. Sox9 progenitors are always associated with branch tips throughout the branching process (Fig. 1.1) and thus must be precisely regulated in at least three aspects. First, the progenitors need to self-renew at a rate that matches the exponentially growing respiratory tree. Second, a controlled fraction of their progeny needs to exit the progenitor pool, forming branch stalks and differentiating into airway and alveolar cells [3, 4]. Third, depending on whether branch tips are split in a symmetric or asymmetric manner to form bifurcation or lateral branches, respectively, the progenitors need to be partitioned between new branch tips accordingly (Fig. 1.2).
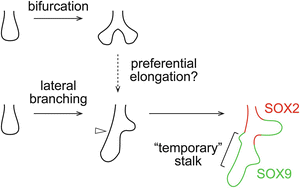

Fig. 1.2
Two modes of new branch formation. New branch tips form via bifurcation or lateral branching. One branch that is preferentially elongated after the symmetric bifurcation may appear as the main branch in the asymmetric lateral branching, suggesting that the two branching modes may be more similar than their final appearance. The main branch contains a “temporary” branch stalk that does not express SOX2 and can initiate new branches (open arrowhead)
Branching morphogenesis does not continue forever, and Sox9 progenitors are depleted after birth (Fig. 1.1). A time course analysis following branch tips along the lobe edge shows that the branch tip-stalk structure disappears after birth as the stalks widen, presumably as a result of differentiation into alveolar ducts and air filling. Meanwhile, Sox9 progenitors persist as clusters at branch tips as late as postnatal day (P) 7 and potentially could continue to divide into smaller clusters, reminiscent of branch tip-splitting during branching morphogenesis. Given their location in the most distal portion of the respiratory tree and beyond alveolar ducts, these Sox9 progenitor clusters presumably differentiate into primary saccules that will be further subdivided to form mature alveoli. Therefore, cessation of branching morphogenesis parallels depletion of Sox9 progenitors.
Spatial: For bifurcation where branch tips form by splitting existing tips, it is intuitive that Sox9 progenitors are located in branch tips, while their differentiating progeny, commonly marked by Sox2 expression, are located in branch stalks. However, for lateral branching where branch tips form on existing stalks, I would like to introduce the concept of “temporary” branch stalks, in which a morphological branch stalk is molecularly a branch tip. Specifically, although “temporary” branch stalks are surrounded by airway smooth muscle [11], they are Sox2 negative as supported by the absence of labeled distal epithelial cells in Sox2 CreER lineage tracing experiments [4]. In other words, before Sox9 progenitors become Sox2-positive “permanent” branch stalks, they remain capable of branching regardless of the tip-stalk morphology (Fig. 1.2). Therefore spatially, Sox9 progenitors are located in the branching region of the developing respiratory tree.
The close temporal–spatial correlation between branching morphogenesis and Sox9 epithelial progenitors suggests that regulation of branching morphogenesis is fundamentally regulation of Sox9 progenitors by intrinsic (epithelial) and extrinsic (mesenchymal) signalings.
Morphological Features of Branching Morphogenesis
The building blocks, branch tips and stalks, form in two modes: bifurcation and lateral branching. Deployment of these branching modes must be regulated in a deterministic manner to form a respiratory tree of a highly reproducible shape and lobation pattern that is specific for each species. Meanwhile, space-filling must also play a role to ensure maximal space utilization without branch collision. This section will focus on the mouse lung whose branching has been studied in depth [12]; and this will be compared with lungs from other species and other branched organs.
Lobation: On a global level, branching morphogenesis is constrained by the size, shape, and arrangement of lobes termed lobation. The mouse lung consists of a single left lobe and four right lobes: cranial, middle, accessory, and caudal lobes. This asymmetry is regulated as part of the bilateral asymmetry of the body plan as situs inversus mutants, such as the Dnahc11 mutant [12], have left-right inverted lungs as well as other internal organs. Closer examination of the branching structure suggests that the mouse lung might be more symmetric than its appearance with the exception of the accessory lobe (Fig. 1.3). Specifically, the caudal lobe can be considered the equivalent of the caudal portion of the left lobe (starting from L.L3) as both consist of a central medially curved lobar bronchus and multiple parallel lateral branches. The axis of the middle lobe parallels the lateral branches of the caudal lobe and thus may be the L.L2 equivalent, while the anterior portion of the cranial lobe is reminiscent of the L.L1.A1 branch. Thus, the main difference between left and right lobes is that individual right lobes are encased and thus separated by a single-layered mesothelium. Future studies of the mesothelium may shed light on mechanisms of normal lobation and abnormal lobe fusion.
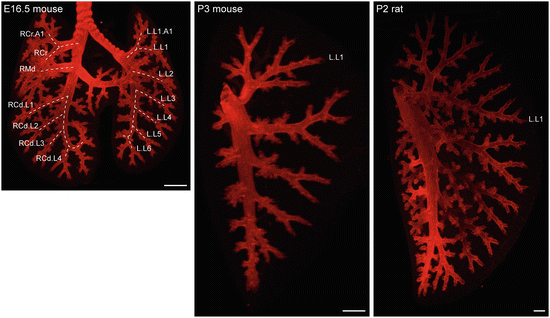

Fig. 1.3
Mouse and rat airways. OPT images of mouse and rat lung or left lobes immunostained for an airway marker SOX2. The mouse lung and left lobe are shown at the same magnification and the rat left lobe is half of that magnification. Scale: 500 um. The branch lineage is labeled to show symmetry between the left and right lobes
The shape of individual lobes is apparently established early in development (Fig. 1.1), raising the interesting question of how branching morphogenesis at different locations advances the lobe surface in a concerted fashion to preserve lobe shape over time. Alternatively, lobe geometry is regulated by other means and feeds into the branching pattern control. Intriguingly, the rat lung is remarkably similar in lobe shape and branching pattern to the mouse lung, but is not simply a scaled-up version as it has more airway branch generations (Fig. 1.3).
Branching subroutines: Careful comparison of hundreds of developmental intermediates has revealed that branching in the mouse lung can be accounted for by three branching subroutines: domain branching, planar, and orthogonal bifurcation [12]. Furthermore, these three subroutines are deployed in three sequences, where orthogonal bifurcation represents a “dead-end” subroutine that will only form rosette-like branches and no longer switch to alternative subroutines [12]. It is interesting to note that, compared to orthogonal bifurcation, domain branching and planar bifurcation, by definition, form longer branch stalks that extend to the lobe edge and can initiate lateral branches. Thus, as discussed below, branch length (or elongation) may be an additional parameter other than branch angle that distinguishes the subroutines.
Dichotomous and monopodial branching: Branching in the mouse lung is often monopodial featuring minor daughter branches on the side of a major parent branch, while branching in the human lung is often dichotomous featuring symmetric daughter branches of comparable size [13]. Although this difference might reflect differential usage of lateral branching versus bifurcation across species, an additional contributing factor may be a difference in branch elongation after, but not during, branch formation. Specifically, differential elongation of one daughter branch after dichotomous branching can form an asymmetric structure reminiscent of the one formed via monopodial branching (Fig. 1.2). Consistent with this possible dichotomous-branching origin of monopodial branches, the major parent branch in monopodial branching is often curved (Fig. 1.3). Furthermore, no gene has been identified whose expression distinguishes dichotomous (bifurcation) and monopodial (lateral) branching, and computer simulation suggests a deterministic role of branch elongation in generating different branching modes [14]. The difference in branch elongation may be ultimately linked to lobe shape, which differs significantly between mouse and human.
Tip morphology in comparison with other branched organs: Compared to a bead-on-a-string design, branching morphogenesis packs a large number of terminal units without incurring a tortuous transport route. Thus, it is used in multiple organs, including the kidney, mammary, and salivary glands. However, the branch tip morphology among organs differs substantially. While branch tips in the lung are bulbous with obvious luminal space, the ureteric tree frequently contains trifurcating branch tips [15]; the salivary gland tips split via clefting due to replacement of cell–cell junctions with cell–matrix interactions [16]; and mammary gland branching requires epithelial-to-mesenchymal transition and migration of a bi-layered epithelium [17]. Although the Drosophila trachea system mediates respiratory function and requires the same fibroblast growth factor (Fgf) signaling as the lung, its branching appears more similar to vertebrate angiogenesis, where selected tip cells lead the rest toward a morphogen source [18]. It is worth noting that in the developing lung, the bulbous branch tip may appear on sections as a large diameter tube, but should not be mistaken as proximal airways, which do not have as large a lumen as those in the adult lung.
Cell Biology of Branching Morphogenesis
The cellular behaviors underlying lung branching morphogenesis are not well understood, in part because existing explant culture systems [19] are limited in recapitulating in vivo development. In particular, cultured lungs are constrained in size by the supporting filters, and the resulting limited branch outgrowth may disrupt the epithelium–mesenchyme cross talk that is essential for normal branching. Furthermore, cultured lungs flatten due to gravity; this disrupts branch shape, morphogen distribution, and potentially intraluminal fluid pressure, a mechanical stimulus possibly involved in branching [20]. In addition, given the exponential nature of branch tip number increase, a small difference in the initial tip number due to variation in developmental timing, coupled with ambiguity in branch tip counting due to altered morphology, may be misinterpreted as treatment effects. Nevertheless, genetic and culture experiments have implicated cell polarity, movement, and matrix interaction in lung branching.
Cell polarity: The developing lung epithelium is a monolayer polarized both along the apical–basal axis and within the monolayer plane. Epithelial-specific deletion of Integrin beta-1 (Itgb1), a major mediator of cell–matrix interaction, completely abrogates branching [21]. The mutant epithelium frequently contains abnormal apical–basally oriented cell division and becomes multilayered with distinct gene expression among layers, reminiscent of a stratified epithelium such as the epidermis [22]. Overactivation of Kras, a small GTPase mediating receptor tyrosine kinase signaling, or loss of Spry1 and Spry2, phosphatases limiting receptor tyrosine kinase signaling, shortens and widens branch tips in association with altered proportions of longitudinal versus circumferential cell divisions [23]. Although computer modeling suggests that abnormal cell division orientation largely explains the observed branch morphology phenotype, a similar mechanism has been proposed for formation of cystic renal tubes but may not be causal to their phenotype in some cases [24].
Cell movement: Although constrained by tight and adherent junctions, the lung epithelium is fluid with frequent interkinetic nuclear migration (INM) along the apical–basal axis and neighbor exchange via intercalation. Best known for its role in neurogenesis, INM positions the nucleus at the basal or apical side during the S or M phase of the cell cycle, respectively, and potentially exposes cells to location-specific signals [25, 26]. Asynchrony in INM among cells makes a monolayered epithelium appear pseudostratified and, depending on cell density, generates bottle-shaped cells with apical and basal thin extensions of varying length [27].
Real-time imaging of cultured lungs has confirmed the association between cell division orientation and branch growth in the longitudinal versus circumferential directions [28]. In addition, no migratory cellular extensions are observed, and instead epithelial cells move via neighbor exchange, a process reminiscent to intercalation during germ band elongation [29]. Unlike intercalation, the net apical surface area of lung epithelial cells can decrease locally via actomyosin contraction, leading to epithelium deformation and branch initiation, in culture and in a Wnt signaling mutant [27, 30]. Future genetic mosaic analyses are necessary to understand whether and how morphogenetic signals, including Wnt and Fgf, regulate cell shape in a cell-autonomous manner. Intriguingly, in the kidney, neighbor exchange occurs even during mitosis via a mitosis-associated cell dispersal process where apically dividing daughter cells move apart and insert back to the epithelium at distant sites [31]. If similar cell behavior also occurs in the lung, it would be interesting to determine how it might be integrated with oriented cell division to regulate branch morphology.
Extracellular matrix (ECM): The lung epithelium is surrounded by a basement membrane that contains fibronectin, collagen, nidogen, and laminin and is thicker around branch stalks than tips [32], suggesting a potential role of ECM in branching morphogenesis. Although such a role is demonstrated in cultured lungs [16, 33], genetic mutants either die before lung formation or exhibit little branching phenotype, presumably due to essential roles of ECM in cell survival or functional redundancy among related genes [34]. Interestingly, several ECM mutants, including those affecting ECM turnover, have abnormal perinatal lung maturation, highlighting the importance of ECM in supporting the air-filled alveolar region [34–37].
Genetic Control of Branching Morphogenesis
Defects in lung branching morphogenesis often lead to respiratory failure and death at birth, a characteristic phenotype that has allowed an effectively forward genetic screen and identification of a number of branching control genes and signalings [1, 38]. Future studies need to assemble the existing parts list into a gene regulatory network, especially with regard to their role in Sox9 epithelial progenitors as they give rise to the entire epithelial tree. This section will summarize the major branching signalings focusing on the epithelial progenitors; highlight importance of nomenclature in phenotypic characterization; and describe available genetic and genomic tools to integrate existing knowledge toward a system-level understanding. Due to the aforementioned limitations of lung explant culture, only genetic mutants are included although caution is also warranted with the interpretation of these models because of the potential nonspecific toxicity of genetic tools [39–41].
Multiple signalings: The primary branching signal utilizes the Fgf10–Fgfr2 pathway. Fgf10 expression is dynamic and localized in the mesenchyme just ahead of growing branch tips [42], and both Fgf10 and Fgfr2 mutants completely lack branching [43, 44]. Epithelial-specific deletion of Fgfr2 leads to a lung with only left and right main bronchi that are devoid of Sox9 progenitors, while epithelial overactivation of Kras, a mediator of receptor tyrosine kinase including Fgf signalings, leads to expansion and persistence of Sox9 progenitors [45]. Such gain-of-function and loss-of-function data support a dominant role of the Fgf signaling in progenitor maintenance.
The Wnt signaling in the developing lung is complex as multiple Wnt genes are present and canonical Wnt activity, depending on the reporter, is detected in either the epithelial or mesenchymal compartment [46]. Wnt2/2b-dependent canonical Wnt signaling controls respiratory fate specification in the foregut [6, 7]. The Wnt7b mutant has a significantly smaller lung with fewer albeit normal looking branches [47]. The Wnt5a mutant also has a smaller lung and a higher density of epithelial cells at late stages, although it is unknown if the total number of branches is increased [48]. Epithelial deletion of Ctnnb1, the mediator of canonical Wnt signaling, decreases the number of Sox9 progenitors whose domain becomes disproportional to that of their Sox2 expressing progeny [4, 49, 50].
Similar complexity exists for the Bmp and Tgf signalings. Although Bmp4 is specifically expressed in the Sox9 progenitors and antagonizes Fgf10 in culture, its in vivo role is still debatable [4, 51, 52]. Similarly, although deletion of Alk3 (Bmpr1a), a Bmp receptor, using Sftpc genetic drivers disrupts branching [52, 53], Shh Cre -mediated deletion of Alk3 has no detectable phenotype (unpublished observation). Only deletion of both Bmp receptors, Alk3 and Alk6, disrupts respiratory fate specification in the foregut [8]. Such redundancy among homologous genes may also apply to the Bmp ligands. Epithelial deletion of Alk5, a Tgf receptor, leads to a smaller lung with fewer and dilated branches [54].
Therefore, existing evidence indicates that the epithelial progenitors receive inputs from Fgf, Wnt, and Tgf signalings. Although not discussed here, the mesenchyme is also precisely regulated by multiple signalings including Fgf9, Wnt, and Shh. Disruption of these will also in turn affect epithelial branching [55–57].
Phenotype nomenclature: Gross histological examination prompted by the characteristic neonatal lethality phenotype often leads to a description of the lung phenotype as hypoplastic or hypercellular, which likely corresponds to defective branching or alveolar differentiation, respectively. Further analyses of such mutants need to define phenotypes using an increasing number of molecular markers available for each process.
Another term commonly used is proximal–distal patterning, which requires clarification and discussion. First, patterning is classically defined as a onetime cell fate choice in a homogeneous field of naïve cells in response to a morphogen gradient, such as the anterior–posterior patterning of the Drosophila embryo [58]. Although the lung epithelium has differential gene expression proximal–distally (e.g., distal Sox9 and proximal Sox2), the proximal region is not the equivalent of the distal region, but instead is made of the continuous influx of the progeny of the distally located Sox9 progenitors. Therefore, proximal–distal patterning, or “proportion” to be exact in the context of branching, reflects cumulative, constant cell fate choices of Sox9 progenitors between self-renewal and differentiation. Such a concept has been explored in recent studies [4, 59].
Second, definition of proximal–distal patterning may be confounded by branch morphology and depends on whether individual branches or the whole lung is compared between the control and mutant. As illustrated in Fig. 1.4, a simple slow branching mutant (type I) will have fewer branches with a normal proximal–distal proportion. Type II mutants can have a disproportionally small distal region as the result of decreased progenitor self-renewal with normal differentiation. Type III mutants are characterized by a smaller lung with fewer branches that are counterintuitively dilated. Due to this abnormal branch morphology, individual branches appear to have an expanded distal region while the total distal region in the whole lung is smaller. The apparent distal bias may be actually accompanied by decreased progenitor self-renewal as supported by the epithelial apoptosis phenotype in the Dicer mutant and the branch dilation phenotype from epithelial cell ablation in the Sftpc–rtTA; tetO–DTA mutant [4, 60]. Therefore, type II and type III mutants may be similar in having decreased progenitor self-renewal and total distal region in the whole lung, while their apparent difference in proximal–distal patterning with respect to individual branches may be confounded by branch dilation. Finally, Type IV mutants can have a disproportionally large distal region as the result of increased progenitor self-renewal with normal or decreased differentiation.
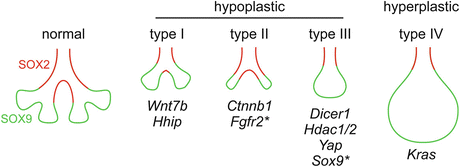

Fig. 1.4




Proximal–distal proportion in mutants with different branch morphology. Type I mutants [47, 57] have fewer normal-looking branches with a normal proximal–distal proportion. Type II mutants [4, 45, 49, 50] have fewer branching containing a smaller progenitor region. Asterisk: the Fgfr2 mutant has no SOX9 progenitors. Type III mutants [45, 59, 60, 70] have fewer branches that are counterintuitively dilated as in the hyperplastic type IV mutant. Compared with the normal lung, type III mutants have a larger distal region with respect to individual branches, but a smaller distal region with respect to the whole lung. Asterisk: the Sox9 mutant has a distal region that does not express SOX9. Type IV mutants [23, 45] have a larger lung with fewer and dilated branches
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


