The History, Physical Examination, and Cardiac Auscultation: Introduction
In the evaluation of patients with definite or suspected heart disease, important information (when sought) can be acquired from the history, physical examination, chest radiography, electrocardiography, and other routine laboratory tests. In fact, this approach has recently undergone the increasing scrutiny of evidence-based clinical diagnosis.1 These data, when integrated properly, facilitate an accurate diagnosis and appropriate decisions regarding therapy in many patients at a relatively low cost. When more information is necessary, additional, more expensive noninvasive cardiac tests such as echocardiography or radionuclide studies are often indicated. In some patients, the general assessment indicates the need for cardiac catheterization and contrast angiography with or without additional noninvasive cardiac testing. For example, the proper approach to certain, but not all, patients with symptomatic coronary artery disease (CAD) may include both coronary arteriography and cardiac catheterization (anatomy and hemodynamics) as well as myocardial perfusion imaging with thallium or technetium sestamibi (extent of inducible ischemia).
Not all patients need every test; the skillful use of low-technology approaches, including the history and general examination (when properly performed), can preclude the need for additional testing or can indicate which of a variety of available sophisticated tests should be selected for a particular patient. This chapter is divided into three sections. The first concerns the proper application of the history and its use to delineate the differential diagnosis in patients who present with certain common cardiovascular symptoms. The second details the essential components of the general physical examination and their usefulness in establishing a likely diagnosis when specific abnormal findings are detected. Finally, the third section focuses on cardiac auscultation.
The History
A carefully obtained history is the cornerstone for evaluating a patient with known or suspected cardiac disease.2 A deliberate, compassionate interview forms the basis for a patient–physician relationship that can continue indefinitely. Unfortunately, the interview can result in adversarial roles for physician and patient if the interviewer appears hurried, shows impatience, fails to establish eye contact, seems to treat dreaded diseases casually, or appears to be unsympathetic. When the medical interview is unsatisfactory because of poor communication and lack of rapport, inaccurate information and often unnecessary testing will be obtained. Also, important facts not revealed during a meticulous initial history are usually not detected later because both the patient and physician become focused on high-technology studies and more aggressive therapeutic interventions.
The patient’s chief complaint, which requires further elaboration and investigation, may not identify his or her most serious problem. Therefore, symptoms other than the patient’s chief complaint must be defined. The interviewer should note all existing symptoms and establish a present illness for each of these.
A medical questionnaire given to the patient well in advance of the interview is useful and can record important data more accurately because of the time thus made available to reflect and check details.
A proper interpretation of the past history is important; the physician should not accept a past event as a fact when the evidence is not well established. Information obtained from family members about the patient’s symptoms and his or her response to the illness is extremely important.
Serious heart disease can occur in patients with mild or no symptoms. The physician must determine whether or not the history obtained is sufficient to support a decision-making process about the patient.2 Although many patients with severe heart disease have no symptoms, others have many symptoms associated with minor or no disease.
Some patients deny symptoms because they cannot accept the reality of the situation, and others purposely withhold information so as not to jeopardize their jobs.2 Some patients overstate their symptoms for personal gain. Elderly patients, sedentary patients, and those with other illnesses can have no symptoms because they are not physically active enough to induce them.
The past history can provide important clues to the presence of cardiovascular disease. A definite history of rheumatic fever can be useful in defining the cause of a heart murmur (see Chap. 75), but a negative history does not exclude it. A history of hypertension in a family member increases the likelihood that the patient has essential hypertension.3 Previous trauma may be the cause of constrictive pericarditis, a thoracic aortic aneurysm, an arteriovenous fistula, and other types of cardiac lesions. A detailed history of the use of medications, addicting drugs, and alcohol, each of which can cause heart disease, is essential. A past history of pulmonary embolism, thrombophlebitis, or systemic embolism should be ascertained.
A history of dental work, some other diagnostic or therapeutic procedure, or recent infection suggests infective endocarditis in a patient with valvular heart disease. Patients often give a history of a heart attack that, in fact, can have been an episode of unstable angina, heart failure, or arrhythmia. The heart attack history often becomes myocardial infarction (MI) in the patient’s medical record unless more information is obtained or documentation of the event is reviewed.
Many patients are referred who have had several catheterizations, percutaneous coronary interventions, and one or more coronary bypass operations in addition to multiple noninvasive tests. A thorough and often time-consuming review of records from other institutions, operative notes, cineangiographic films, and noninvasive studies will often provide an accurate assessment of the patient’s current status without the unnecessary repetition of expensive and potentially risky procedures.4
Past and present therapeutic regimens must be reviewed carefully. Previous treatment programs may have been inappropriate or suboptimal. Drugs used for the treatment of patients with cardiovascular diseases have potential side effects that can produce both cardiovascular and noncardiovascular symptoms (see Chap. 95).
Multiple risk factors for developing coronary heart disease (CHD) have been identified, including age, male gender, hypertension, hypercholesterolemia, a low level of high-density lipoprotein (HDL) cholesterol, cigarette smoking, diabetes, and a family history of premature atherosclerosis (see Chap. 50).
Information from previous health evaluations should be sought. Patients have often been examined for military service, athletics, or insurance, and they might have been told of a heart murmur or hypertension on those occasions5 or were rejected for medical insurance or military service. Many patients have never had a careful examination of the cardiovascular system.
The increasing hemodynamic burden of pregnancy can cause an otherwise marginally compensated cardiac patient to become symptomatic (see Chap. 97). Specific inquiry should be made about heart failure, edema, dyspnea, or prescribed prolonged periods of bedrest during pregnancy. Many normal women have had a murmur detected during pregnancy. A history of illicit parenteral drug use should raise the suspicion of infective endocarditis, especially in a febrile patient (see Chap. 86). Cocaine can cause coronary artery vasospasm and also raise myocardial oxygen demand by increasing heart rate and blood pressure. Angina, MI, and sudden cardiac death after cocaine use have been well documented (see Chap. 95).
A history of moderate to excessive alcohol consumption, an enlarged heart on a prior chest radiograph, periods of rapid weight gain or loss, and other illnesses can provide important information.
A family history of congenital heart disease indicates a higher risk of a congenital heart lesion (see Chap. 84). If the patient’s mother gives a history of rubella during the first few months of pregnancy, this increases the likelihood that the patient has patent ductus arteriosus, pulmonic valve stenosis, coarctation of the pulmonary arteries, or atrial septal defect (ASD).
Although many of the common cardiovascular diseases are sporadic, genetic transmission has been documented in a rapidly increasing number of disease entities. These are detailed elsewhere (see Chap. 82).
Chest pain or chest discomfort is the foremost manifestation of myocardial ischemia and results from a disparity between myocardial oxygen demand and coronary blood flow (CBF) in patients with CAD. The most common causes of myocardial ischemia are coronary atherosclerosis, coronary vasoconstriction, and coronary artery thrombosis, the latter occurring particularly in patients with acute coronary syndromes (ACS) such as acute MI and unstable angina (see Chaps. 58 and 59). An increase in myocardial oxygen consumption (MVO2) or demand ischemia, a decrease in or inadequate blood flow (supply ischemia), or their combination can be responsible for anginal chest pain (see Chap. 53).
The mechanism responsible for cardiac pain is not clearly understood. Nonmedullated small sympathetic nerve fibers that parallel the coronary arteries are thought to provide the afferent sensory pathway for angina; these enter the spinal cord in the C8 to T4 segments. Impulses are transmitted to corresponding spinal ganglia and then through the spinal cord to the thalamus and cerebral cortex. Angina pectoris, similar to other pain of visceral origin, is often poorly localized and is commonly referred to the corresponding segmental dermatomes.
The differential diagnosis of chest pain is extensive.4 In addition to angina pectoris and MI, other cardiovascular diseases, gastrointestinal diseases, psychogenic diseases, neuromuscular diseases, and diseases of the pulmonary system must be considered (Table 14–1). An accurate interpretation of the cause and significance of chest discomfort is critically dependent on a carefully taken history. Important, clinically relevant information can be missed if the overenthusiastic use of noninvasive or invasive diagnostic methods replaces rather than augments direct physician–patient communication (See Chap. 63).
| 1. Angina pectoris or myocardial infarction |
| 2. Other cardiovascular causes |
| a. Likely ischemic in origin |
| (1) Aortic stenosis |
| (2) Hypertrophic cardiomyopathy |
| (3) Severe systemic hypertension |
| (4) Severe right ventricular hypertension |
| (5) Aortic regurgitation |
| (6) Severe anemia/hypoxia |
| b. Nonischemic in origin |
| (1) Aortic dissection |
| (2) Pericarditis |
| (3) Mitral valve prolapse |
| 3. Gastrointestinal |
| a. Esophageal spasm |
| b. Esophageal reflux |
| c. Esophageal rupture |
| d. Peptic ulcer disease |
| 4. Psychogenic |
| a. Anxiety |
| b. Depression |
| c. Cardiac neurosis |
| d. Self-gain |
| 5. Neuromusculoskeletal |
| a. Thoracic outlet syndrome |
| b. Degenerative joint disease of the cervical or thoracic spine |
| c. Costochondritis (Tietze syndrome) |
| d. Herpes zoster |
| e. Chest wall pain and tenderness |
| 6. Pulmonary |
| a. Pulmonary embolus with or without pulmonary infarction |
| b. Pneumothorax |
| c. Pneumonia with pleural involvement |
| 7. Pleurisy |
The original subjective description of angina pectoris by William Heberden3 in the late 18th century has not been surpassed. It is quoted in Chap. 63. Angina pectoris is defined as chest pain or discomfort of cardiac origin that usually results from a temporary imbalance between myocardial oxygen supply and demand.6 It can occur only with exertion or spontaneously at rest; various subtypes are defined in Chap. 63. The quality of the chest discomfort is usually described as “tightness,“ “pressure,“ “burning,“ “heaviness,“ “aching,“ “strangling,“ or “compression.“ The patient is usually able to describe a deep rather than a superficial origin of the pain. Because the qualitative description of the pain is greatly influenced by the patient’s intelligence, education, and social and cultural background, a delineation of the other characteristics of the chest discomfort is often extremely important in evaluating the symptoms appropriately. The most important of these characteristics are the precipitating factors for the onset of pain, its mode of onset and duration, its pattern of disappearance, and its location. Classically, the discomfort is induced by exercise, emotion, eating, or cold weather.
A recognizable pattern of reproducibility of chest pain by certain activities is an important characteristic of angina. Often, patients develop pain with exertion after meals, and there is a greater tendency for arm work (a greater degree of isometric exercise) to produce distress. Occasionally, angina will dissipate despite continued exercise (the walk-through phenomenon) or will not occur when a second exercise effort is undertaken that previously produced chest discomfort (warmup phenomenon). Both circumstances can be attributed to the opening of functioning coronary arterial collaterals during the initial myocardial ischemia. This is consistent with ischemic preconditioning as described elsewhere (see Chap. 53).
Angina commonly occurs after the patient has eaten a heavy meal or when he or she is excited, angry, or tense. Whereas cold showers increase blood pressure and heart rate, hot showers cause an augmented cardiac output in response to vasodilation. Either can precipitate angina after exercise. The chest pain during any activity is often made worse by the use of tobacco. All of the hemodynamic changes caused by the use of nicotine increase myocardial oxygen demand.
Angina pectoris characteristically has a crescendo pattern at onset and “builds up.“ Pains, often described as “shooting“ or “stabbing,“ that reach their maximum intensity virtually instantaneously are often not angina but are of musculoskeletal or neural origin. Angina is usually relieved within 5 to 20 minutes by rest with or without the use of vasodilator drugs such as nitroglycerin (TNG), although sublingual TNG or TNG spray characteristically hastens relief. Failure to obtain relief with rest or TNG suggests another cause of pain or actual impending MI. The reproducible relief of chest pain in an appropriate time frame (usually within 10 minutes) is strong evidence favoring ischemia. A trial of TNG can be a useful diagnostic strategy. Patients with angina pectoris are usually classified functionally from class I to class IV (Table 14–2), depending on the amount of activity necessary to induce chest pain.
| I. Ordinary physical activity, such as walking and climbing stairs, does not cause angina. Angina results from strenuous or rapid or prolonged exertion at work or recreation. |
| II. Slight limitation of ordinary activity. Walking or climbing stairs rapidly, walking uphill, walking or stair climbing after meals, in cold, in wind, or when under emotional stress or only during the few hours after awakening. Walking more than two blocks on the level and climbing more than one flight of ordinary stairs at a normal pace and under normal conditions. |
| III. Marked limitations of ordinary physical activity. Walking one to two blocks on the level and climbing more than one flight under normal conditions. |
| IV. Inability to carry on any physical activity without discomfort—anginal syndrome can be present at rest. |
Localizing the site of chest discomfort provides additional information as to its cause. Anginal pain is ordinarily retrosternal or felt slightly to the left of the midline beside or partly under the sternum. It is rarely isolated to the cardiac apex in the inframammary region. The chest pain of myocardial ischemia tends to radiate bilaterally across the chest into the arms (left more than right) and into the neck and lower jaw. Occasionally, radiation to the back or occiput is noted. In the arms, the pain passes down the ulnar and volar surface to the wrist and then only into the ulnar fingers, rarely into the thumb or down the outer (extensor) surface of the arm, which has a different dermatome pattern. Pain can occasionally be felt only in the arm or can start in the arm and radiate to the chest. Noting the patient’s gestures in characterizing and localizing the site of pain can be useful. One or two clenched fists held by the patient over the sternal area (Levine sign) is much more indicative of ischemic pain than is a finger pointed to a small, circumscribed area in the left inframammary region. The latter is likely an indicator of psychogenic pain.
The duration of chest pain can also be a useful differentiating feature. Angina pectoris rarely lasts less than 1 minute or more than 20 minutes in the absence of MI or persistent arrhythmias. Most patients with angina report prompt relief in less than 5 minutes after cessation of activity or with the use of sublingual or spray TNG. Carotid sinus massage should be performed only in the absence of extracranial occlusive cerebrovascular disease as indicated by carotid bruits or decreased carotid arterial pulsations and with careful auscultatory monitoring of the heart rate. The Valsalva maneuver can also relieve anginal pain by decreasing myocardial wall stress because of the reduced venous return and left ventricular (LV) volume accompanying the increase in intrathoracic pressure. Associated symptoms—such as nausea, vomiting, faintness, fatigue, or diaphoresis—often accompany severe episodes of myocardial ischemia. Severe myocardial ischemia often produces marked dyspnea caused by a large increase in LV diastolic filling pressure, sometimes producing an angina equivalent in the absence of chest discomfort.7,8
Linked angina is a term applied to definite episodes of angina in patients with established CAD caused by gastrointestinal factors unrelated to an increase in cardiac work. Episodes are typically induced by stooping or occur after eating; they can be mimicked by esophageal acid stimulation, which can reduce CBF.
No consideration of myocardial ischemia as a likely cause of chest discomfort is complete without carefully considering the chest pain in the context of known risk factors for CAD (see Chap. 50).
Angina pectoris should be considered a symptom and not a specific disease. Coronary arteriographic studies have demonstrated that more than 90% of patients with chest pain precipitated by exercise and relieved by rest have angiographic evidence of significant CAD.9 However, other diseases can be associated with classic angina pectoris.
Several reports have described certain patients with typical exertional chest discomfort and arteriographically normal coronary arteries. These patients are more likely to be women; have fewer coronary risk factors; and have variable responses to various antianginal agents, including TNG. Although the underlying cause of this condition remains unsettled, the life expectancy of these patients appears no different from that of an age- and gender-matched population without chest discomfort.
Some evidence suggests that abnormal function of small coronary arteries can cause limited CBF responses to stress or pharmacologic vasodilators in a subset of patients with anginal chest pain despite angiographically normal coronary arteries (microvascular angina). In the past, investigators arguing for or against the existence of this syndrome have often used the term syndrome X to describe their patient cohort. Syndrome X appears to include a heterogeneous group of patients with a wide spectrum of chest pain and a variety of hypersensitive vascular and smooth muscle constrictor responses. Multiple research studies continue in an effort to explain syndrome X. The term syndrome X is a poor substitute for chest pain in patients with normal large vessel coronary arteriography. It is likely to represent a polyglot of disorders. It must be distinguished from the metabolic syndrome (X) of insulin resistance (glucose intolerance), hypertension, hyperlipidemia, and upper body obesity. The latter is often associated with severe vascular disease and frequently abnormal coronary arteriography. Some patients with classic angina but near-normal contrast laminography and angiography fail to demonstrate areas of plaque formation because of coronary artery remodeling. Intracoronary ultrasonography often demonstrates plaque of various size, volume, and location when CHD is present.
Some patients with CAD experience angina at rest as a complication or an isolated clinical manifestation of ischemic heart disease.10 Myocardial ischemic pain at rest more likely results from an acute reduction in CBF than from an increase in MVO2. Possible causative factors include isolated coronary artery spasm or embolism, coronary artery spasm superimposed on coronary atherosclerosis (a common occurrence), and coronary thrombosis with spontaneous thrombolysis. In patients with progressive coronary atherosclerosis, however, ischemic rest pain also can result from intermittent arrhythmias that increase MVO2 or decrease CBF or from labile hypertension, with its increased systolic wall stress. Chest pain at rest can occur only as nocturnal angina. In addition, nocturnal angina (also known as angina decubitus) can be produced by the increase in LV wall stress and as a result of the redistribution of the intravascular blood volume in the recumbent position.
The relative hypercapnia and acidosis that occur during sleep can also contribute to nocturnal angina (see Chap. 73). The diagnosis of sleep apnea is often overlooked in patients with angina, heart failure, and arrhythmia. Nocturnal angina has been accompanied by concomitant rapid eye movement sleep patterns on the electroencephalogram, which can be associated with augmented sympathetic discharge increasing or causing coronary constriction11-14 (see Chap. 63).
The quality of pain with rest angina is usually similar to that of exertional angina, but the discomfort can be more severe and its duration longer. In addition, angina at rest is commonly associated with nausea, vomiting, and diaphoresis. The onset of shortness of breath during or after the beginning of chest discomfort suggests that the pain is caused by extensive myocardial ischemia and results from an acute elevation of LV filling pressure secondary to the development of a large, transiently ischemic myocardial segment. Such patients commonly have multivessel occlusive CAD on arteriography.
It is important to emphasize that a significant number of individuals with clinically important CAD will have no symptoms, minimal symptoms, or atypical symptoms. This is particularly true of the elderly population, diabetic patients, and women.7 For example, dyspnea on exertion in the absence of chest pain may be an anginal equivalent that reflects acute pulmonary venous congestion in an individual with a large area of reversible ischemia-induced LV dysfunction or transient mitral regurgitation caused by papillary muscle dysfunction.
Chest pain or discomfort resulting from MI is qualitatively similar to angina at rest. Differentiating the pain resulting from ischemia and that caused by MI is often impossible based on the history alone. Pain associated with ACS is usually more severe and longer lasting than anginal pain and is often associated with nausea, vomiting, and diaphoresis (see Chaps. 58 and 59). In addition, MI or other ACS is frequently accompanied by symptoms of sustained LV dysfunction (dyspnea, orthopnea) and evidence of autonomic nervous system hyperactivity (tachycardia, diaphoresis, bradycardia). Painless or atypical presentations of MI, however, occur in up to 40% of patients, particularly in diabetic and elderly patients. Thus, determination of serial serum enzymes, isoenzymes, and other serum biomarkers (eg, troponin I or T); providing evidence of myocardial necrosis; and serial electrocardiograms (ECGs) indicating myocardial injury are necessary to establish the diagnosis (see Chaps. 58 and 59).
There are two groups of cardiovascular diseases causing chest pain that is not caused by coronary atherosclerosis (see Table 14–1). The first group consists of cardiac diseases causing myocardial ischemia–related angina in the absence of CAD. Ischemia is caused by hemodynamic changes associated with an inadequate CBF in relation to a normal or increased myocardial oxygen demand. Among these are aortic valve stenosis (see Chap. 76), hypertrophic cardiomyopathy (HCM; see Chap. 35), and systemic arterial hypertension (see Chap. 69), in which LV systolic pressure and LV wall tension are greatly increased or LV hypertrophy is present. Chest pain caused by myocardial ischemia may also occur with severe aortic regurgitation (AR) (see Chap. 76). The large LV volume load increases LV end diastolic pressure and the reduced diastolic perfusion pressure results in a relatively inadequate CBF. Occasionally, very severe anemia or hypoxia can also produce myocardial ischemia as a result of inadequate oxygen blood supply even in the absence of associated CAD. Both also can increase angina in the presence of obstructive CAD. In addition, severe right ventricular (RV) systolic hypertension, as often occurs with pulmonic stenosis (PS), or pulmonary hypertension (PH), can cause exertional angina, presumably on the basis of RV subendocardial ischemia.
A second group of cardiac diseases causing chest pain not usually caused by myocardial ischemia includes pericarditis (see Chap. 85), aortic dissection (see Chap. 106), and mitral valve prolapse (MVP) (see Chap. 77). Pericarditis is a relatively common cause of chest pain. It is most often sharp and penetrating in quality; patients often obtain relief by sitting up and bending forward. The cardinal diagnostic feature of pericardial pain is its frequent worsening by changes in body position, during deep inspiration, and occasionally on swallowing. The chest discomfort can radiate to the shoulders, upper back, and neck because of irritation of the diaphragmatic pleura, which is innervated through the phrenic nerve by fibers originating in sympathetic ganglia C3 to C5. Therefore, the chest discomfort associated with pericarditis is caused predominantly by parietal pleural irritation. Occasionally, the pain of acute benign, presumptive viral pericarditis can mimic that observed in acute MI. Importantly, the most common cause of pericarditis in middle- aged or older people is acute MI. The pericarditis usually occurs several days after the myocardial necrosis and must be distinguished from recurrent infarction or ischemia. Pericarditis can also be a cause of chest pain after cardiac surgery and can be a complication of aortic dissection, with leakage into the pericardium.
Aortic dissection (see Chap. 106) can be misdiagnosed on initial presentation as an acute MI; indeed, MI is a recognized complication of aortic dissection. The pain with dissection, however, is usually of sudden onset compared with that of myocardial ischemia, which builds in intensity with time.11 Patients frequently characterize the pain as excruciating, having a tearing quality, and commonly localized to the interscapular area. The discomfort can radiate widely into the neck, back, abdomen, flanks, and legs and can migrate, depending on the location and progression of the aortic dissection and the amount of arterial luminal compression. Neurologic symptoms and signs may occur when dissection involves the cerebral arteries. With the exception of patients with Marfan syndrome or idiopathic cystic medial necrosis, most patients with aortic dissection have a history of long-standing systemic arterial hypertension or evidence of it on physical examination or by ECG (left ventricular hypertrophy [LVH]).
Psychogenic chest discomfort is a common recurrent chest pain that can be difficult to separate from angina pectoris, particularly when it occurs in patients with multiple risk factors for CAD or in otherwise asymptomatic patients with well-documented CAD. The most common psychogenic cause of chest discomfort is anxiety (see Chap. 96). Psychogenic chest pain is often described as sharp or stabbing, localized to the left inframammary area, and usually sharply circumscribed. Descriptors such as “stabbing“ or “lightning-like“ can be used to describe extremely short (<1 minute) episodes of pain. At times, the pain can persist for many hours or several days. Patients often note psychogenic pain at rest. Also, nonvocal communication—such as a flat or worried facial expression, retarded motor activity, and hand wringing—can indicate underlying depression. Observation of the patient during pain that occurs spontaneously or during exercise testing often provides insight into a potential psychogenic etiology. Patients with anxiety often have multiple complaints. Associated symptoms—such as air hunger, circumoral paresthesias, globus hystericus, and multiple somatic complaints—may suggest a neurasthenic personality or hyperventilation syndrome.
Pain originating in the gastrointestinal tract, particularly that of esophageal origin, is commonly confused with ischemic chest pain.12-14 Diffuse esophageal spasm, a neuromuscular motor disorder of the esophagus characterized by chest pain, is the extracardiac condition most frequently confused with angina pectoris. Esophageal spasm can occur at any age but is more common in 50- to 60-year-old individuals. The pain is usually retrosternal; can be burning, squeezing, or aching in quality; and often radiates to the back, arms, and jaw. It usually begins during or after a meal and can last minutes or hours. The pain can be relieved by TNG, which also relaxes esophageal smooth muscle. A useful diagnostic feature in esophageal spasm is its frequent association with pain as a result of swallowing, dysphagia, and the regurgitation of gastric contents. The diagnosis of diffuse esophageal spasm is based on the history, the exclusion of cardiac and musculoskeletal causes of chest pain, and the demonstration of abnormal esophageal motility on cine-esophagograms or by esophageal manometry.
Reflux esophagitis results from mucosal irritation produced by failure of the lower esophageal sphincter to prevent regurgitation of highly acidic gastric contents into the distal esophagus. The pain is usually epigastric or retrosternal, burning in quality, and frequently precipitated by the recumbent position or by bending over. Heartburn and regurgitation often occur after meals or ingestion of coffee or after postural changes. Patients are often awakened by chest discomfort caused by acid reflux occurring in the recumbent position. Dysphagia may result from stricture formation secondary to long-standing esophageal reflux. An upper gastrointestinal x-ray series can demonstrate hiatal hernia, but this does not establish the diagnosis of esophagitis or esophageal reflux. Esophagoscopy and esophageal biopsy can demonstrate mucosal lesions and are useful for assessing the severity of inflammation and for excluding malignancy. Sphincter incompetence can be documented by the use of esophageal manometry. Esophageal acid perfusion testing (Bernstein test) often provokes the patient’s characteristic symptoms, and distal esophageal pH monitoring will detect gastroesophageal reflux.15
Acute esophageal rupture, a serious and often rapidly lethal event, causes severe retrosternal pain secondary to the chemical mediastinitis produced by acidic gastric contents. Spontaneous rupture usually results from a prolonged bout of vomiting or retching after a heavy meal. The pain varies in location depending on the rupture’s site and position. The diagnosis is based on symptoms and signs of mediastinal air after vomiting or esophageal instrumentation.
Although peptic ulcer disease and biliary colic are less commonly confused with chest pain of cardiac origin, myocardial ischemic pain can occasionally be described as burning in character and located near the epigastrium.
Diseases involving the neuromuscular and skeletal systems can cause pain affecting dermatomal patterns similar to those occurring with angina pectoris. The thoracic outlet syndromes, in which various neural and vascular structures are compressed, can produce symptoms that are sometimes confused with cardiac chest pain. Although compression of the neurovascular bundle by a cervical rib or the scalenus anterior muscle can cause discomfort radiating to the head and neck, the shoulder region, or the axilla, most patients experience pain in the upper extremity ulnar distribution resulting from somatic nerve compression. The presence of associated paresthesias and pain unrelated to physical exercise as well as the worsening of discomfort with certain body positions are useful differentiating characteristics.
Tietze syndrome, or idiopathic costochondritis, is an occasional cause of anterior chest wall pain that is aggravated by movement and deep breathing. Reproducing the chest pain syndrome by direct pressure over the involved costochondral junction or the relief of pain after local infiltration with lidocaine is a helpful diagnostic maneuver. Degenerative arthritis of the cervical and thoracic vertebrae can cause bandlike pain confined to the chest, neck, or back that often radiates to the arms. Radiologic evidence of degenerative changes involving the cervical and thoracic vertebrae is often found in asymptomatic elderly patients. The production or exacerbation of pain by various postures, movement, sneezing, or coughing is more useful in the diagnosis of chest discomfort caused by vertebral disease.
The preeruptive stage of herpes zoster can be characterized by bandlike chest pain over one or more dermatomes. The advanced age of the patient; additional symptoms of malaise, headache, and fever; the presence of hyperesthesia of the involved area on physical examination; and the eventual eruption of typical lesions 4 or 5 days after the onset of symptoms will result in the correct diagnosis. Chest wall pain and tenderness can also occur for unknown reasons. The discomfort can be reproduced by pressure over the painful area and by movements of the thorax such as bending, twisting, or turning.
The syndrome of acute massive pulmonary embolism with its associated acute PH and low cardiac output occasionally can simulate acute MI because myocardial ischemia can be present in both conditions. The quality of chest pain can be identical to that observed in patients with nonradiating ischemic chest pain or can be pleuritic. The associated signs of severe dyspnea, tachypnea, and intense cyanosis accompanied by profound anxiety and agitation, however, favor the diagnosis of pulmonary embolism9-12 (see Chap. 72). Measurements of arterial blood gases, CT angiography, or pulmonary ventilation/perfusion scans, and if needed, pulmonary arteriography will establish the correct diagnosis.
Other pulmonary conditions associated with chest discomfort, such as pneumothorax, are rarely confused with ischemic chest pain because of additional characteristic clinical features. Spontaneous pneumothorax usually occurs in otherwise healthy men in the third and fourth decades of life. The clinical presentation is usually characterized by the abrupt onset of agonizing unilateral pleuritic chest pain associated with severe shortness of breath. The plain or expiratory chest film provides the definitive diagnosis. Chest pain associated with pneumonias of various causes as well as pulmonary infarctions as a consequence of pulmonary embolus may result from pleural irritation. The discomfort is sharp, varies acutely with breathing, and is frequently accompanied by a reduced inspiratory effort. Associated signs of pulmonary parenchymal infection or infarction usually indicate the underlying diagnosis.
Intermittent claudication of the lower extremities caused by peripheral atherosclerosis (see Chap. 109) can present as discomfort during exercise in the arch of the foot, calf of the leg, thighs, hips, or gluteal region. Acute arterial occlusion in the lower extremities caused by systemic embolism can cause the sensation of hypesthesia and or pain. The pain of Raynaud disease can be noted in the fingers after exposure to cold, with pallor of the fingers before the sensation of pain. Pain and swelling of lower extremities can be caused by thrombophlebitis.
Head pain secondary to myocardial ischemia can be felt in the jaw, hard palate, and cheek and sometimes deep in the ear canals. The pain of temporal arteritis, commonly localized to the temporal area, is often associated with abnormal vision and polymyalgia rheumatica. A severe headache can be present in patients with uncontrolled hypertension (see Chap. 68).
Pain in the abdomen, often localized to the midabdomen and lower portion of the back, can be produced by an expanding or rupturing atherosclerotic abdominal aneurysm. Abdominal angina caused by vascular disease of the mesenteric arteries is discussed in Chap. 106. The liver is often painful and tender in severe right-sided heart failure, with worsening of the pain during activity.
Various types of joint pain can be associated with heart disease (see Chaps. 75 and 89). Rheumatic fever, rheumatoid arthritis, lupus erythematosus, psoriatic arthritis, ankylosing spondylitis, gonococcal arthritis, Reiter syndrome, and Lyme disease can be associated with valvular, myocardial, or pericardial disease.
Dyspnea is defined as difficult or labored respiration or the unpleasant awareness of one’s breathing. A clue to the cause is obtained from the factors that precipitate or relieve it. Chronic dyspnea may be caused by heart failure, pulmonary disease, anxiety, obesity, poor physical fitness, pleural effusions, and asthma. Acute dyspnea may occur with acute pulmonary edema, hyperventilation, pneumothorax, pulmonary embolism, pneumonia, and airway obstruction.
Dyspnea on effort, a frequent symptom, is usually caused by congestive heart failure, chronic pulmonary disease, or physical deconditioning (see Chap. 31). A recent or dramatic increase in dyspnea is more likely to be caused by the development of heart failure than by lung disease. When heart and lung disease coexist, however, determination of the relative contribution of pulmonary and cardiac dysfunction to dyspnea can be very difficult.
Cheyne-Stokes respiration is a form of periodic breathing characterized by cycles beginning with shallow respirations that increase in rate and depth to significant hyperpnea followed by decreasing rate and depth of respiration and then a period of apnea that can last 15 seconds or longer. This form of respiration occurs in advanced congestive heart failure and in some forms of central nervous system disease. Cheyne-Stokes respiration often occurs during sleep without the patient’s awareness and is often reported by others.
Orthopnea results from an increase in hydrostatic pressure in the lungs that occurs with assumption of the supine position. It consists of cough and dyspnea in some patients with LV failure or mitral valve (MV) disease and necessitates the use of two or more pillows on lying down. Patients with severe obstructive lung disease, especially acute asthma, also cannot lie flat comfortably.
Paroxysmal nocturnal dyspnea (PND) is the occurrence of dyspnea during sleep, commonly 2 to 3 hours after going to bed, which is relieved by assuming the upright position. Episodes can be mild, or they can be severe with wheezing, coughing, gasping, and apprehension.1 Some episodes progress to pulmonary edema. The probable mechanism for this relatively specific symptom of left-sided heart failure is the increase in central blood volume in the supine position.
A dry, unproductive cough occurring with effort or at rest may be related to the pulmonary congestion associated with heart failure (see Chap. 27). Although dyspnea is usually present, cough can dominate the clinical picture. Whereas the cough that accompanies acute pulmonary edema is often associated with frothy, pink-tinged sputum, the sputum associated with chronic bronchitis is usually white and mucoid. Sputum associated with pneumonia is often thick and yellow and because of pulmonary infarction can be bloody, as can the sputum associated with cancer of the lung or bronchiectasis. Cough can also be caused by angiotensin-converting enzyme inhibitors or much less commonly angiotensin receptor blockers.
Recurrent coughing caused by heart failure is often thought to be caused by bronchitis, and patients with chronic bronchitis can cough more when heart failure ensues. Patients with a high pulmonary blood flow caused by congenital left-to-right shunts are subject to pulmonary infection. Patients with a high pulmonary venous pressure (eg, mitral stenosis [MS]) are more vulnerable to pulmonary edema when they have viral pneumonitis than are patients with normal pulmonary venous pressure.
Hemoptysis occurs in many cardiac disorders. Posterior epistaxis as a result of systemic hypertension can cause blood-streaked sputum; patients on anticoagulants can have epistaxis that mimics hemoptysis. Bright red pulmonary venous blood from rupture of submucosal pulmonary venules can be expectorated by patients with pulmonary venous hypertension caused by MS or severe LV failure. Darker blood or clots often occur with pulmonary emboli.
Pink, frothy sputum can be produced during acute pulmonary edema. Blood-streaked sputum is a feature of the winter bronchitis of MS. Massive hemoptysis with exsanguination can follow rupture of an aortic aneurysm or one of the cardiac chambers into the bronchial tree. Rupture of a pulmonary artery by the balloon of an indwelling pulmonary artery catheter can cause abrupt, severe hemoptysis in hospitalized patients.
Wheezing associated with dyspnea can be caused by lung or heart disease. If the symptoms have developed recently in an adult older than 40 years of age, other clues indicating heart disease (cardiac asthma) should be sought.
Edema is a common symptom or finding in patients with right-or left-sided heart failure. Fluid retention in heart failure results from increased venous pressure and abnormal activity of salt-retaining hormones (see Chap. 30). In an average-sized person, 5 to 10 lb (2.3-4.5 kg) of excess fluid is required for edema to become apparent; a history of recent weight gain will often correlate with deterioration in clinical status. The amount of weight loss in response to treatment for heart failure in the past will relate to the severity of the problem. Minor degrees of edema are evident only after a period of dependency of the legs and decrease after rest. Presacral edema can be most obvious when the patient has been on bedrest. Although edema of cardiac origin can progress to anasarca, cardiac edema rarely involves the face or upper extremities. Persistent edema in the legs from which veins were harvested at the time of bypass surgery is common. Other causes of edema—such as varicosities, obesity, tight girdle, renal insufficiency, or cirrhosis with hypoproteinemia—must be considered. A patient with chronic congestive heart failure can detect edema of the ankles and lower legs during the day and note that it diminishes during the night. It is important to ascertain whether edema of the extremities preceded or followed dyspnea on effort. The calcium antagonists can produce bilateral edema of the lower legs. Edema may occur in one or both legs after the harvesting of veins for conduits in patients undergoing coronary artery bypass grafting (CABG) surgery.
Patients will be aware of ascites because of increased abdominal girth. Previously comfortable trousers or skirts can no longer fit. Bending at the waist is uncomfortable, with ill-defined abdominal fullness. Patients with severe edema caused by congestive heart failure may develop ascites; however, ascites is particularly common in patients with constrictive pericardial disease, sometimes occurring before peripheral edema becomes obvious (see Chap. 85). Ascitic fluid is formed when elevated venous pressure leads to transudation of fluids from the serosal surfaces.
Fatigue and weakness can be caused by many conditions and therefore are not specific symptoms for heart disease. The most common cause of these symptoms when isolated is anxiety and depression. Anemia, thyrotoxicosis, and other chronic disease states can be associated with fatigue and weakness.
When a patient with heart disease is volume overloaded or when there is pulmonary congestion caused by heart disease, the patient is likely to complain of dyspnea. With vigorous diuretic therapy, this complaint can be replaced by symptoms of fatigue and weakness, probably related to inadequate cardiac output (see Chap. 28). As congestive heart failure worsens, fatigue can replace dyspnea as the major symptom. β-Blockers used to treat angina or hypertension and LV dysfunction often cause fatigue and lethargy. When starting β-blockers to treat heart failure, “start low and go slow.“ Hypotension or hypokalemia caused by diuretics can result in fatigue and weakness, as can relative hypovolemia caused by the use of angiotensin-converting enzyme inhibitors.
Most normal individuals are intermittently aware of their heart action, particularly at times of physical and emotional stress. When the heart action is more vigorous than usual or its perception is unpleasant, the term palpitation is appropriate. The patient may complain of a “pounding,“ “stopping,“ “jumping,“ or “racing“ in the chest. Palpitation is frequently a benign symptom without any serious cardiac disease present; at other times, it can indicate a potentially life-threatening condition. Simple premature beats can be perceived as a “floating“ or “flopping“ sensation in the chest caused by the more forceful beat that occurs after the pause following the premature beat. Sometimes a transient feeling of fullness in the neck (caused by cannon A waves) is perceived with premature beats. Certain patients perceive almost every premature beat, but others are totally unaware of frequent or advanced arrhythmias. A report of skips or irregularity during uninterrupted sinus rhythm is common. Generally, thin, tense individuals are likely to be more aware of their cardiac activity than others. Individuals with and without arrhythmias often are aware of their cardiac activity when they first lie down to sleep, especially if they lie on their left side.
Rapid heart action of a paroxysmal tachycardia usually begins and terminates abruptly and causes a pounding sensation in the chest. Patients often indicate whether the tachycardia is regular or irregular and can be able to tap out the rate and rhythm of the episode (see Chap. 39). Chest pressure suggesting angina can occur with an episode of tachycardia even in young, healthy patients without CAD. Patients with CAD, however, often develop severe angina with a sustained arrhythmia because of increased Depending on the rate and mechanism of the arrhythmia, faintness and syncope can be described during questioning. Nevertheless, sustained ventricular tachycardia may occur in the setting of serious underlying cardiac disease without a significant compromise in hemodynamics (see Chap. 41). Syncope caused by tachyarrhythmias may occur without the patient being aware of palpitations.
Cardiac syncope (fainting) is defined as the transient loss of consciousness caused by inadequate cerebral blood flow secondary to an abrupt decrease in cardiac output (see Chap. 5). Near syncope refers to the clinical situation in which the patient feels dizzy and weak and tends to lose postural tone but does not lose consciousness. In assessing a patient with syncope, one determines if there were precipitating factors, premonitory symptoms, injury with the episode, seizure activity or incontinence, or a postictal state. Injury during an episode suggests a sudden profound loss of body tone and increases the likelihood of more serious causes. Brief, unsustained seizure activity can occur with syncope caused by a cardiac arrhythmia.
The patient can be incontinent during cardiogenic syncope, but an aura, sustained tonic-clonic movements, tongue biting, and confusion or drowsiness after the event are more characteristic of syncope caused by central nervous system disease. In contrast, return of consciousness to the alert state is prompt after reversal of the arrhythmia causing cardiac syncope. The common faint (vasovagal syncope) results from bradycardia and hypotension caused by excessive vagal discharge. It is often associated with some precipitating event such as a heavy meal in a warm room and has brief premonitory signs and symptoms such as nausea, yawning, diaphoresis, and sometimes the feeling of decreased hearing or vision. The results of head-up, tilt-table testing indicate a vasovagal mechanism in some patients with syncope who do not have premonitory symptoms. After a fainting episode, the patient may be pale and diaphoretic and have a slow heart rate. A history of similar episodes during the preceding several years is common in patients with vagal syncope.
A hypersensitive carotid sinus can cause syncope. A history of episodes during an activity such as shaving, wearing a tight collar, or extreme turning of the head can occur but is unusual even when a sensitive carotid sinus is shown to be the cause of syncope. Syncope after urination (micturition syncope) can occur at the time of rapid decompression of a distended bladder, which typically occurs after a period of sleep. Paroxysms of coughing, usually in patients with underlying pulmonary disease, can result in syncope. Very fast or slow arrhythmias can decrease the cardiac output enough to cause alterations in consciousness, ranging from abrupt profound syncope to mild lightheadedness. Stokes-Adams syncope is caused by intermittent complete heart block, sinus arrest, or ventricular tachyarrhythmias. It is characterized by an abrupt loss of consciousness without warning, a variable period of unconsciousness (seconds to minutes), and then a rapid return of normal mental status without amnesia or a postictal state.
In the presence of severe LV outflow obstruction (aortic stenosis or HCM), loss of consciousness with effort can occur. Syncope can be caused either by the heart’s inability to increase its output in response to the peripheral vasodilatation that occurs during exercise or by a tachyarrhythmia. Intermittent obstruction of a cardiac valve by an intracavitary tumor or thrombus is a rare cause of syncope that can occasionally be precipitated when the patient changes position.
Many normal subjects experience transient lightheadedness with rapid changes in position. This is more common in older patients because the ability of the peripheral vasculature to respond is attenuated with aging. Postural hypotension is a well-defined cause of fainting or dizziness that usually occurs when the individual is upright and often just after rising from a supine or sitting position. Possible causes include peripheral neuropathy, autonomic dysfunction, volume depletion, or drug side effects.
Patients with decreased cardiac output secondary to heart failure can become mentally confused and disoriented. Such symptoms also can be caused by hypoxia, drugs that are invariably prescribed for such patients, and renal or hepatic failure.2 A completed stroke can be caused by a lacunar infarct, cerebral hemorrhage, cerebral arterial thrombosis, or a cerebral embolus (see Chap. 107). A transient cerebral ischemic attack is commonly caused by an embolus. The embolus can originate in an atheromatous ulcer in the carotid artery system or the aortic arch; be related to infective endocarditis, a recent MI, atrial fibrillation, or clots on a prosthetic valve; or originate in the leg veins and pass through a patent foramen ovale to the brain.
A patient with cardiogenic shock or with a severe tachyarrhythmia who also has considerable intracranial or extracranial vascular disease can develop such severe cerebral hypoxia that coma occurs. Hypoxic encephalopathy can follow cardiac resuscitation and occasionally occurs after cardiopulmonary bypass for cardiac surgery. A cerebral abscess can occur in patients with congenital heart disease and a right-to-left shunt.2
Fever with chills is common in patients with infective endocarditis. Symptoms of fever, chills, or sweats in any patient with a heart murmur should lead one to suspect infective endocarditis (see Chap. 86). A history of valvular heart disease is not a prerequisite because previously normal valves become infected. A history of recent dental work, genitourinary surgery, or illicit drug use increases the suspicion of infective endocarditis. Fever can accompany rheumatic fever or pericarditis. An intracardiac tumor (myxoma) can produce systemic symptoms in the absence of infection. Low-grade fever in a patient with heart failure can be a sign of pulmonary emboli. Excessive sweating can occur in patients with severe AR or acute MI. Diaphoresis is often a sign of congestive heart failure in infants.
Hoarseness can occur in patients with an aortic aneurysm that involves the left recurrent laryngeal nerve. MS can occasionally produce hoarseness caused by the pressure of a large pulmonary artery on the recurrent laryngeal nerve. Pericardial effusion can be related to myxedema, which can be associated with a coarse, low-pitched voice. Hoarseness and loss of voice can occur after the use of an endotracheal tube during cardiac surgery.
Many patients with angina pectoris caused by CAD erroneously attribute their symptoms to indigestion or heartburn. Also, patients with heartburn, esophageal reflux, and esophageal spasm may believe they have angina pectoris. Hiccups occasionally occur in patients with MI and during the postoperative period after cardiac surgery. Dysphagia can occur in patients with progressive systemic sclerosis, an aortic arch anomaly, or an extremely large left atrium (LA).
Anorexia, nausea, and vomiting can occur as a result of cardiac glycoside excess or inferior MI Hepatomegaly associated with tricuspid valve disease or severe right-sided heart failure can cause right upper quadrant epigastric pain and fullness as well as anorexia. Abdominal pain caused by visceral ischemia or infarction can occur in a patient who has had a period of very low cardiac output.
Although cyanosis is a sign rather than a symptom, patients or family members may describe cyanosis during the history. Cyanosis is a bluish color of the skin or mucous membranes caused by excess amounts of reduced hemoglobin. Approximately 4 g of reduced hemoglobin is required for cyanosis to be apparent (see Chap. 83). Severely anemic patients will not exhibit cyanosis. A distribution of cyanosis involving the mucous membranes as well as the periphery (central cyanosis) is caused by the admixture of venous blood at the level of the heart or great vessels. A patient or a family member can detect that the cyanosis is more intense in the feet than in the hands. This differential cyanosis suggests a right-to-left shunt through a patent ductus arteriosus in a patient with Eisenmenger physiology. Peripheral cyanosis does not involve the mucous membranes but is the result of slow peripheral flow with accumulation of excess reduced hemoglobin in the setting of circulatory failure, shock, or peripheral vasospasm.
Jaundice can be detected by a patient or by a member of the family. As a rule, hepatic congestion caused by heart failure will not produce jaundice. Hemolysis of red blood cells can occur in patients with prosthetic valves and can produce jaundice.
A history of flush of face and trunk, sometimes accentuated by alcohol, should lead one to search for the other signs and symptoms of carcinoid heart disease (see Chap. 86). Cardiomyopathies caused by hemochromatosis should be considered in a patient with diabetes whose skin color has changed from normal to bronze. A slate-like color of the skin, hands, and nose can develop in patients who take amiodarone.
The most common causes of insomnia are mental conflict, emotional disturbances, and depression. Heart failure, however, can also cause insomnia. Patients with Cheyne-Stokes respirations (see preceding section on Respiratory Symptoms) can sleep during the apneic phase and wake during the hyperpneic phase of the condition. Occasionally, patients with pulmonary congestion caused by heart failure have insomnia before they develop nocturnal dyspnea. Central and obstructive sleep apnea are discussed elsewhere (see Chap. 73).
Several classifications have been proposed and used for the systematic and reproducible grading of disability caused by cardiac disease. Although the complete New York Heart Association method of classifying cardiac diagnoses, originally proposed many years ago, is not widely used now, the portion of the classification that concerns functional capacities16 in heart failure is still commonly used (Table 14–3). Although the Canadian Cardiovascular Society’s grading system for angina (see Table 14–2) is more widely used for patients with chest pain, both classifications continue to be used in the medical literature and in clinical practice, particularly as criteria for the inclusion of heart patients in multicenter clinical trials.
| Class 1: No symptoms with ordinary physical activity |
| Class 2: Symptoms with ordinary activity; slight limitation of activity |
| Class 3: Symptoms with less than ordinary activity; marked limitation of activity |
| Class 4: Symptoms with any physical activity or even at rest |
The Cardiovascular Physical Examination
Important information concerning patients with heart disease is often obtained by a meticulous physical examination, which includes a general inspection of the patient, an indirect measurement of the arterial blood pressure in both arms and one or both lower extremities, an examination of central and peripheral arterial pulses, an evaluation of the jugular venous pressure and pulsations, palpation of the precordium, and cardiac auscultation.
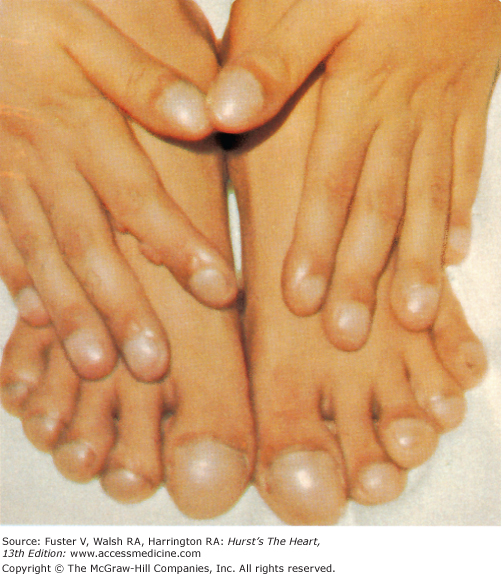
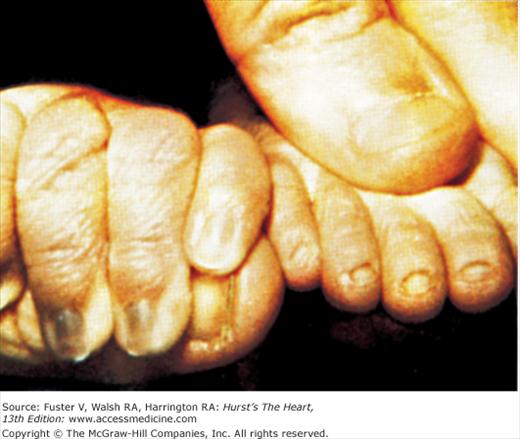
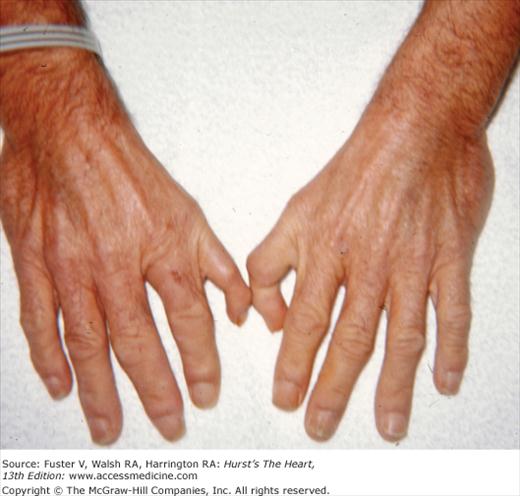
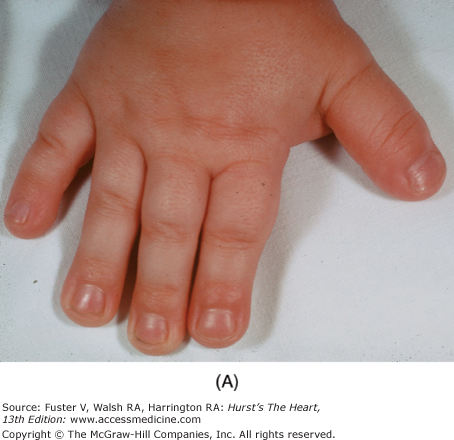
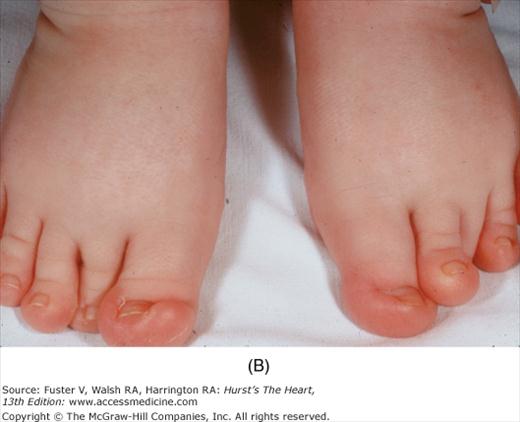
Bedside examination begins with a careful general appraisal of the patient. This visual inspection can provide important information leading to the cause of cardiovascular disease. Clues to the diagnosis of underlying congenital heart disease can be obtained from careful observation of the thorax and extremities. Bilateral prominence of the anterior chest with bulging of the upper two-thirds of the sternum is commonly present in children with a large ventricular septal defect (VSD). A unilateral bulge at the fourth and fifth intercostal spaces at the lower left sternal border is found in adults with VSD. Scoliosis can be present in individuals with cyanotic congenital heart disease. Underdeveloped musculature of the lower extremities compared with the upper extremities occurs with coarctation of the aorta. Clubbing of the digits and cyanosis of the skin or nails suggest congenital heart disease with right-to-left shunting of blood (Fig. 14–1). Differential cyanosis provides information about anatomy. Cyanosis and clubbing of the toes associated with pink fingernails of the right hand and cyanosis and clubbing of the left hand are caused by a patent ductus arteriosus with normally related great vessels and a reversed shunt as a result of PH, with the patent ductus arteriosus delivering cyanotic blood to the left arm and lower extremities (Figs. 14–2 and 14–3). The same color pattern results from interruption of the aortic arch and a patent ductus arteriosus delivering desaturated blood to the legs. However, if the right subclavian artery arises proximal to the aortic obstruction, the right hand can be pink and the left hand cyanotic. When an anomalous right subclavian artery originates from the descending aorta, both hands are cyanotic. Cyanosis of the fingers greater than in the toes indicates complete transposition of the great vessels with preductal coarctation or complete interruption of the aortic arch, PH, and a reverse shunt through a patent ductus arteriosus, in this case delivering oxygenated blood to the lower extremities.
Syndromes Associated with Congenital Heart Disease
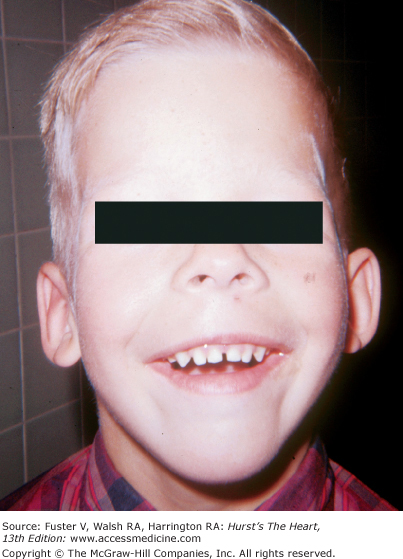
The presence of any congenital somatic abnormality should prompt a search for congenital heart disease. The Ellis-van Creveld syndrome is a heritable form of dwarfism characterized by short extremities, polydactyly, dysplastic teeth and nails, and multiple frenula binding the upper lip to the alveolar ridge (Fig. 14–4). More than 50% of these individuals have heart disease, usually a large ASD or a single atrium.
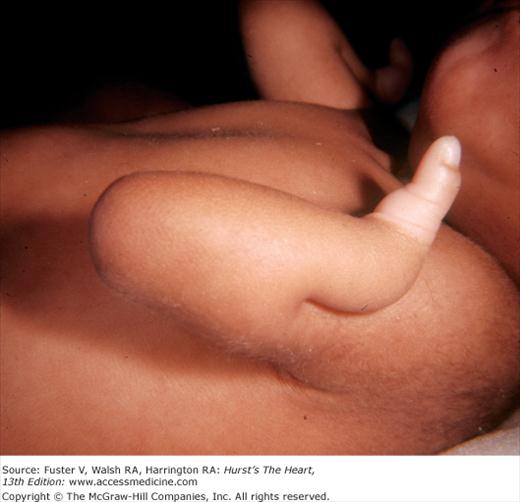
Thrombocytopenia–absent radius (TAR) syndrome includes bilateral radial aplasia with a persistent thumb and thrombocytopenia and can be associated with an ostium secundum ASD or tetralogy of Fallot. Holt-Oram syndrome, an autosomal dominant condition, combines an ASD or other congenital heart disease with an abnormal thumb (Fig. 14–5). In Laurence-Moon–Bardet-Biedl syndrome, mental retardation, polydactyly, obesity, retinitis pigmentosa, and hypogonadism occur with a variety of congenital heart diseases.
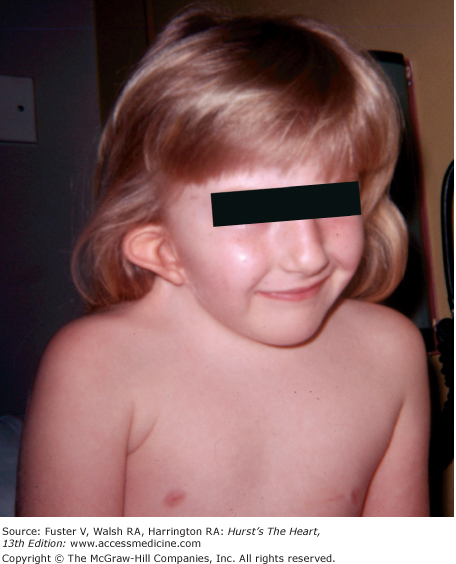
Cornelia de Lange syndrome is characterized by bushy, confluent eyebrows; downward-slanting eyes; a small mandible; low-set ears; hirsutism; long eyelashes; a broad, flat, upturned nose; severe growth and mental retardation; and a peculiar chicken-wing extremity with a single thumb-like digit (Fig. 14–6). A VSD, patent ductus arteriosus, PS, anomalous venous return, or ASD can be present.
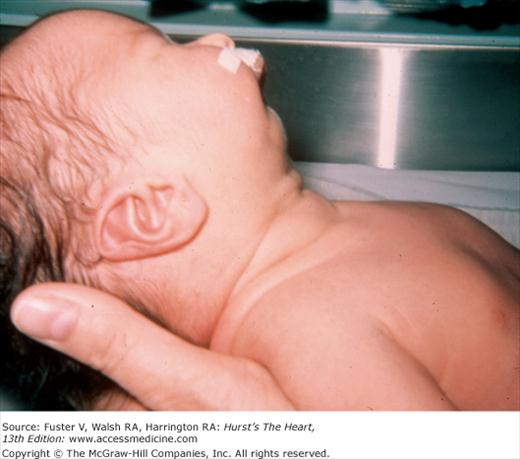
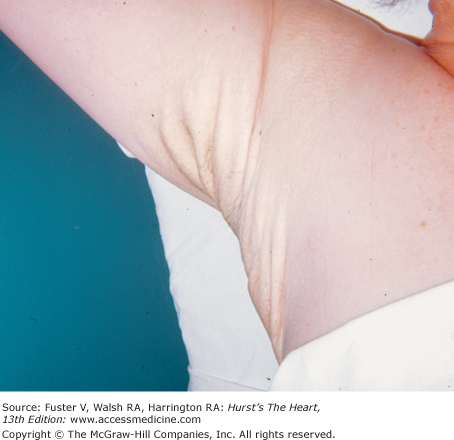
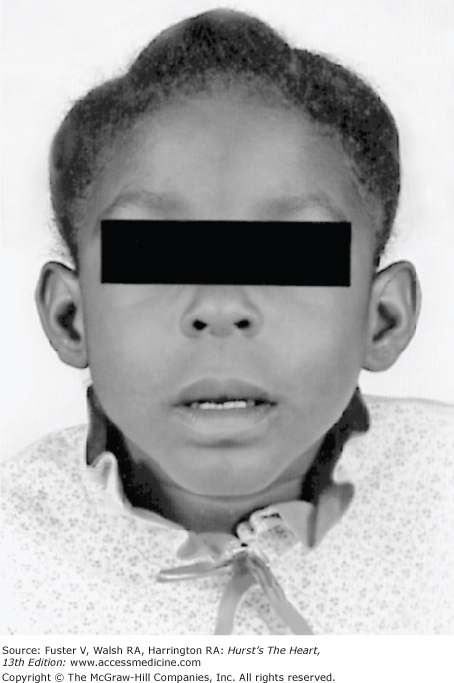
There is an increased incidence of congenital heart disease in children with a cleft palate or lip. In Pierre Robin syndrome (Fig. 14–7), the cleft palate is associated with a hypoplastic mandible causing a shrew-like face. Patients with the Ehlers-Danlos syndrome (Fig. 14–8) have hyperextensible joints and hyperelastic and friable skin often associated with arterial dilatation and rupture, AR, or MVP. Patients with osteogenesis imperfecta have brittle bones, blue sclera, short legs, and an increased incidence of aortic and mitral regurgitation (MR). Patients with pseudoxanthoma elasticum have degeneration of dermal elastic fibers and retinal angioid streaks and can develop AR and CAD (Fig. 14–9).
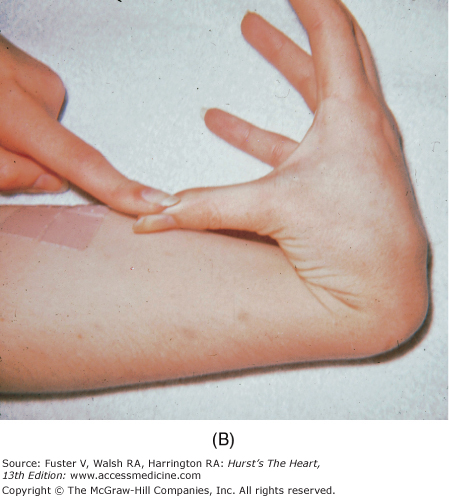
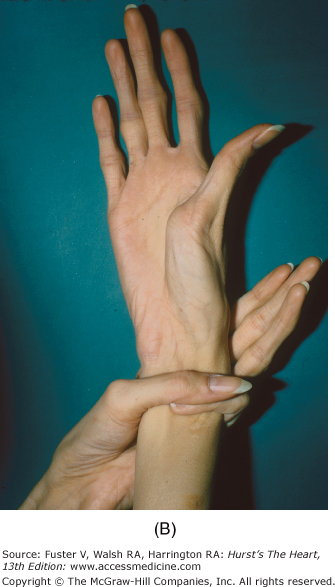
Marfan syndrome, an autosomal dominant disorder, is suggested by skeletal features such as increased height, long fingers, lax joints, kyphoscoliosis, pectus excavatum or carinatum, an elongated face, a high-arched palate, and flat feet (Fig. 14–10). The legs are disproportionately long, and the arm span can exceed the height. When a patient clenches the fingers around their flexed thumb, the thumb protrudes past the ulnar side of the hand (thumb sign). The wrist can be encircled by grasping it with the fifth finger and thumb of the other hand (wrist sign) (see Fig. 14–9B). Other findings include bilateral subluxation of the lens, severe myopia, and blue sclera. Patients with Marfan syndrome usually have MVP, MR, a calcified mitral annulus, and chordal rupture. AR is a consequence of a dilated aortic root, prolapse of the aortic cusps, or aortic dissection.
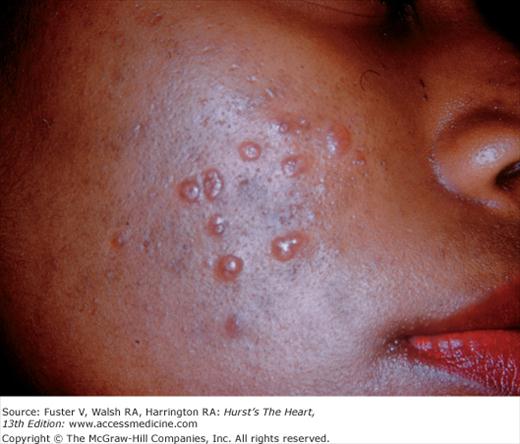
Patients with Morquio syndrome are identified by their short stature, short neck, barrel chest, broad mouth, short nose, widely spaced teeth, and cloudy cornea. In Scheie syndrome, growth retardation, sternal protrusion, facial abnormalities, and cloudy cornea are present. In Fabry disease, angiokeratomas (purplish pinpoint skin lesions) occur on the lips, underarm, buttocks, scrotum, and penis (Fig. 14–11). Cardiomyopathy, ischemic heart disease, and conduction defects are associated with this sex-linked recessive disorder in which there is a genetic deficiency of the enzyme α-galactosidase A.
Many chromosomal abnormalities cause congenital heart disease. The well-recognized characteristics of Down syndrome (trisomy 21) (Fig. 14–12) include a small head, shallow orbits, epicanthal folds, low-set ears, widely spaced eyes (hypertelorism), Brushfield white spots of the iris, a protruding tongue, transverse palmar creases, and mental retardation. Congenital heart disease occurs in 40% to 60% of individuals with Down syndrome; a VSD or endocardial cushion defect are the most frequent. Klinefelter syndrome is characterized by gynecomastia, small testicles, a eunuchoid appearance, tall stature, and long extremities septum, proximum and secundum. ASDs have been described.
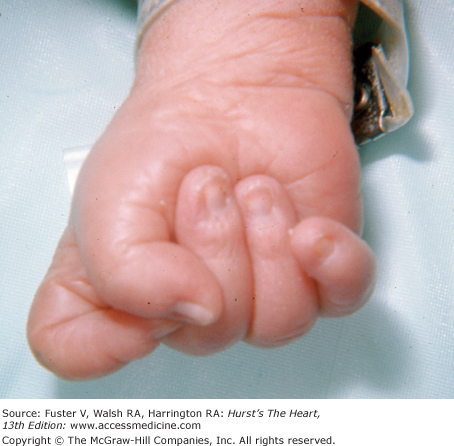
Congenital heart disease of varied types is common in trisomy 13 and trisomy 18 syndromes. In trisomy 13 syndrome, the child has a cleft palate and lip; the ocular tissue and the nose can be missing. Polydactyly, retroflexible thumbs, transverse creases, hyperconvex narrow nails, and flexion of the fingers and hands are characteristic of this syndrome. The features of trisomy 18 syndrome are a small, triangular mouth with a receding chin, small mandible, webbed neck, and tightly clenched fists with the index finger overlapping the third finger and the fifth finger over the fourth (Fig. 14–13).
A low hairline; low-set ears; deafness; small jaw; and short, webbed neck are physical findings common to both Turner syndrome and Klippel-Feil syndrome. Turner syndrome (Fig. 14–14) also includes short stature, a broad chest with widely spaced nipples, epicanthal folds, widely spaced eyes, pigmented moles, ptosis, clinodactyly (curved fifth finger), and a shortened fifth finger. Coarctation of the aorta, aortic stenosis, and HCM are the cardiovascular considerations. Klippel-Feil syndrome can cause facial asymmetry, cleft palate, torticollis, scoliosis, deafness, strabismus, and hydrocephaly. VSD is the most common cardiac disorder.
Sporadic disorders are associated with congenital heart disease. The VATER association includes vertebral defect, anal (imperforation), tracheoesophageal fistula, and radial and renal dysplasia. A ventricular defect occurs in 80% of these patients. Asplenia syndrome is associated with a high incidence of complex congenital heart disease. Cardiovascular malformations are found in 15% to 25% of newborns with omphalocele.
Teratogenic effects resulting in congenital heart disease can be alcohol related; the result of rubella during pregnancy; or induced by phenytoin, thalidomide, or lithium. From 30% to 40% percent of children born to alcoholic mothers are affected with the fetal alcohol syndrome. These children have an undeveloped-appearing central face because of maxillary hypoplasia, a small and upturned nose, an indistinct or smooth philtrum, micrognathia, and a thin upper lip and vermilion (Fig. 14–15). ASDs and VSDs are most common. The teratogenic effects of rubella syndrome include cataracts, deafness, microcephaly, patent ductus arteriosus, pulmonic valvular or arterial stenosis, and ASD.
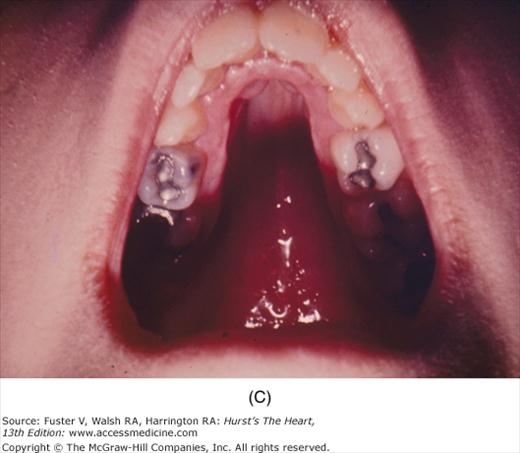
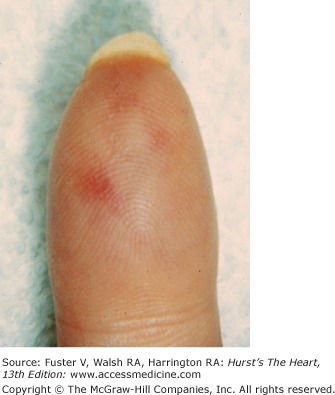
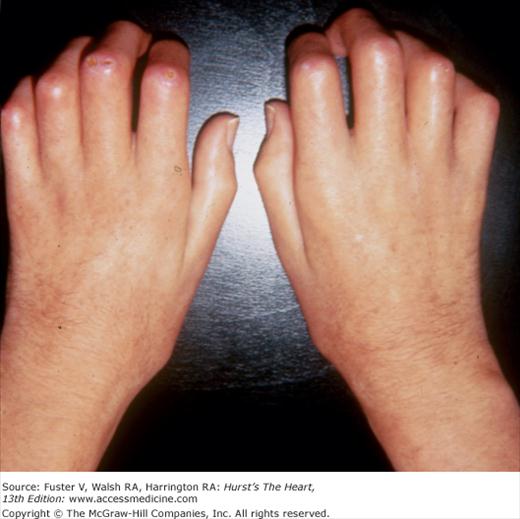
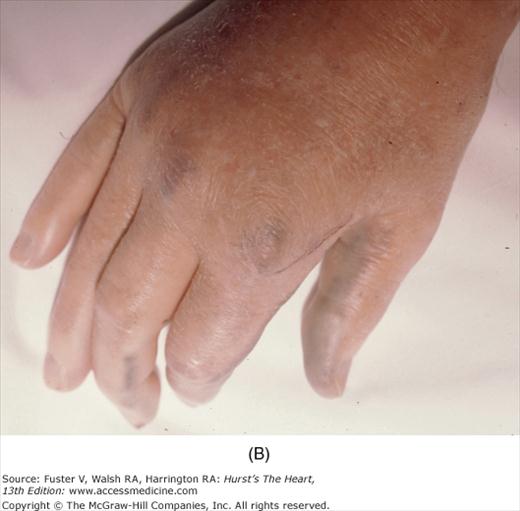
The cutaneous manifestations of infective endocarditis (see Chap. 86) include Osler nodes, Janeway lesions, clubbing of the fingers (Fig. 14–16), splinter hemorrhages of the nails, and petechiae. Osler nodes are reddish purple, tender nodules typically found in the distal pad of the finger or toe (see Fig. 14–16). Janeway lesions are hemorrhagic, nontender, and involve the palms or soles. Splinter hemorrhages are linear, black, and appear in the distal third of the fingernail.
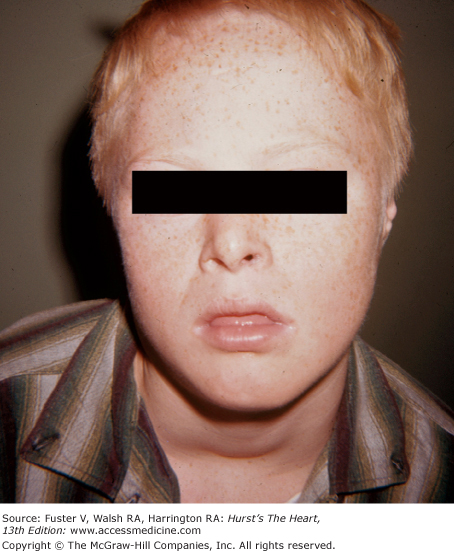
Pulmonic stenosis can be part of Noonan syndrome, Turner syndrome, Rubinstein-Taybi syndrome, rubella syndrome, multiple-lentigines syndrome, pulmonary valve dysplasia, or Watson syndrome. In Noonan syndrome, the characteristic findings include ptosis, low-set ears, downward-slanting eyes, a webbed neck, hypertelorism, a low posterior hairline, short stature, mental retardation, normal chromosomes, and a dysplastic pulmonic valve (Fig. 14–17). Broad toes and thumbs (Fig. 14–18); a slanting forehead; a thin, beaked nose; and large, low-set ears are seen in Rubinstein-Taybi syndrome.
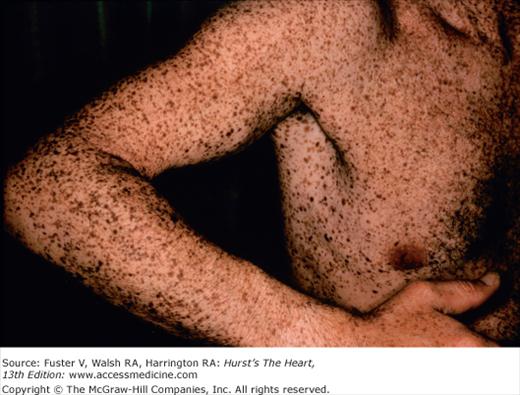
Multiple lentigines syndrome is identified by the presence of multiple dark brown macules varying in size from pinpoint to 5 cm (Fig. 14–19). They cover the entire body but are most heavily concentrated on the neck and upper thorax.
Carcinoid syndrome can present as intense flushing of the face and a chronic cyanotic hue. Telangiectasia can be present. Stenosis or regurgitation of the tricuspid or pulmonic valves can result when hepatic metastases are present. When a patent ductus arteriosus, lung metastases, or a patent foramen ovale is present, and the left-sided heart valves may also be affected.
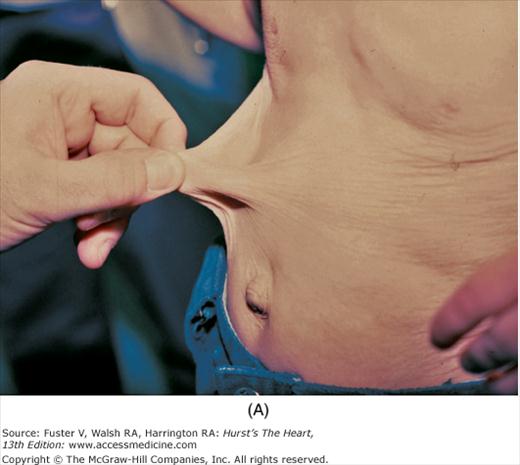
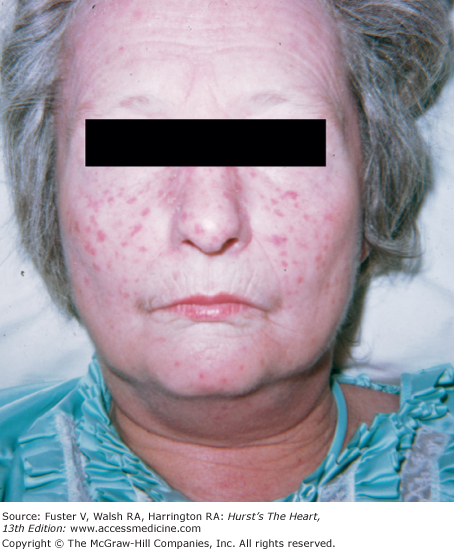
In scleroderma, there is tightening of the skin of the fingers and then the hands, forearms, upper chest, and face. Subcutaneous tissue and skin creases disappear. Flexion contractures of the fingers can cause a claw-like hand deformity (Fig. 14–20). Raynaud phenomenon is an early manifestation. CREST syndrome (calcinosis cutis, Raynaud phenomenon, esophageal motility disorder, sclerodactyly, and telangiectasia) is a variant of scleroderma (Fig. 14–21). Valvular changes, including thickening of the edges of the mitral, aortic, and tricuspid valves, as well as thickening and shortening of the mitral chordae, are rarely significant.
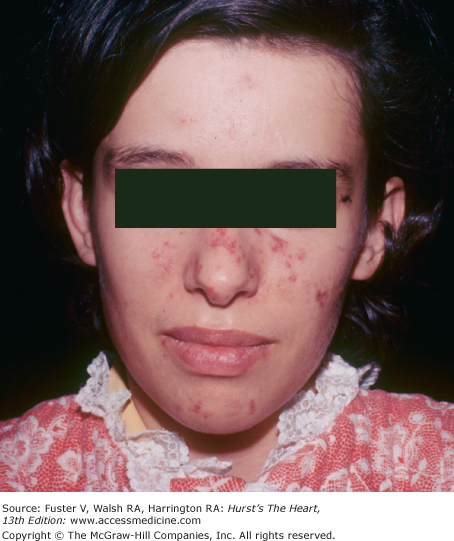
Joint disease is often associated with cardiac valvular disease and can occur with systemic lupus erythematosus (SLE), rheumatoid arthritis, rheumatic fever, polychondritis, ankylosing spondylitis, alkaptonuria, and Whipple disease. In SLE, the joint inflammation is usually symmetric and nondeforming. Typical skin lesions include an erythematous, scaling eruption over the cheeks and bridge of the nose; circumscribed reddish purple plaques; telangiectasia; and patchy hair loss (Fig. 14–22). Verrucous endocarditis is a pathologic finding. Hemodynamically significant atrioventricular (AV) valve dysfunction may occur in long-standing SLE.
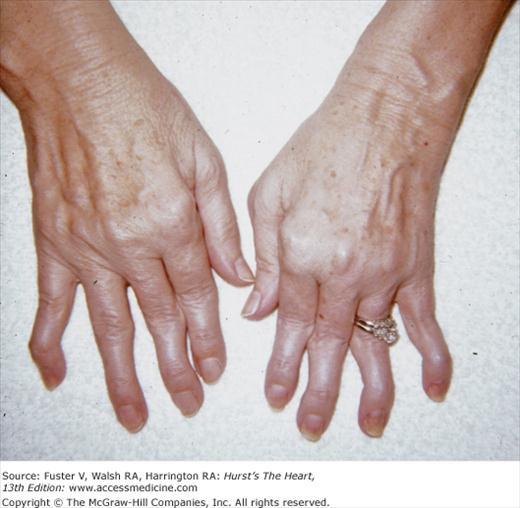
In patients with rheumatoid arthritis, the wrists, shoulders, knees, ankles, and elbows can become inflamed. Advanced disease results in ulnar deviation of the fingers and flexion of the distal interphalangeal joints with hyperextension of the proximal interphalangeal joints, producing a swan-neck deformity and a Z-shaped configuration of the thumb (Fig. 14–23). Granulomatous aortic or MV disease with regurgitation is most common in patients who are seropositive and have subcutaneous nodules or classic rheumatoid deformities. Rheumatic fever should be suspected in patients with erythema marginatum and migratory polyarthritis involving the large joints. Marked ulnar deviation, suggesting rheumatoid arthritis, can be caused by repeated attacks of rheumatic fever and is known as Jaccoud arthritis. In contrast to rheumatoid arthritis, the fingers can be moved freely back into a correct alignment.
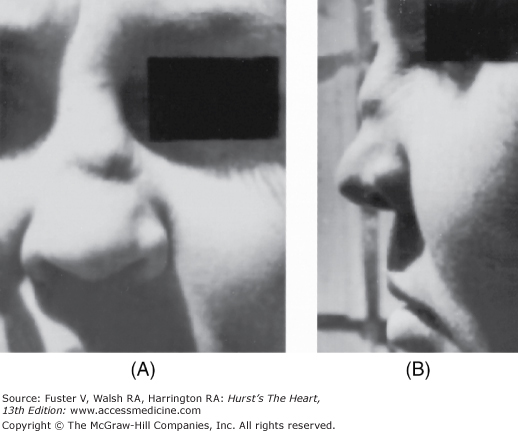
Polychondritis causes an inflammatory destruction of cartilage, resulting in a saddle-shaped collapse of the nose or a cauliflower ear. Aortic root dilatation and (rarely) dissection are associated (Fig. 14–24).
Chronic synovitis of the spine occurs in patients with ankylosing spondylitis. Patients with advanced disease are bent forward, unable to stand upright, and walk with a stiff and halting gait (Fig. 14–25). Aortic regurgitation as a result of the thickening and shortening of the aortic cusps from perivascular inflammation and fibrosis, MR, and complete heart block may occur.
Whipple disease is suggested by polyarthritis, abdominal pain, and diarrhea. Aortic and MR and endocarditis are known complications. Aortic or mitral valvular disease can be caused by an accumulation of homogentisic acid in alkaptonuria (Fig. 14–26). Blue-black, stiff ears and joints are important clues to this inherited disorder of tyrosine metabolism.
Patients with the MVP syndrome can have a straight thoracic spine, pectus excavatum, scoliosis, micromastia, and joint laxity (Fig. 14–27). Systolic and rarely diastolic murmurs have been described with chest wall deformities as a result of a straight-back syndrome and pectus excavatum that can impinge on or displace the heart.
HCM that can be concentric or asymmetric has been associated with Friedreich ataxia, Turner syndrome, Noonan syndrome, Fabry disease, neurofibromatosis, and multiple lentigines syndrome. Friedreich ataxia is a spinocerebellar degenerative disorder that results in a broad-based, lurching gait; impaired vibration, position, and joint sense; and incoordination. Kyphoscoliosis and pes cavus (high instep, retraction of the toes at the metatarsophalangeal joints, and hammer toes) are two important physical signs (Fig. 14–28).
Cor pulmonale can be secondary to PH caused by kyphoscoliosis, restrictive lung disease, scleroderma, upper airway blockade by enlarged tonsils and adenoids, or sleep apnea syndrome.
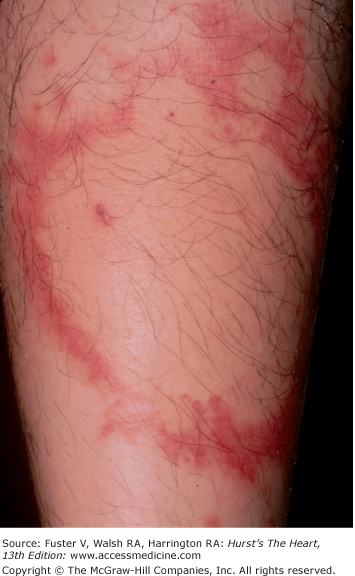
Myocarditis (see Chap. 71) occurs with SLE, rheumatic fever, Reiter syndrome, Kawasaki disease, Lyme arthritis, and occasionally Whipple disease. In Reiter syndrome, conjunctivitis and hyperkeratotic coalescing lesions encrusted on the soles and palms, known as hyperkeratosis blenorrhagicum, are associated with arthritis and urethritis (Fig. 14–29). Kawasaki disease begins with fever; nonexudative conjunctivitis; dry, fissured lips; cervical adenopathy; and a strawberry tongue. Later, the palms and soles become indurated and purplish red and then peel. A widespread erythematous rash can appear and then desquamate. Lyme arthritis, caused by the spirochete Borrelia burgdorferi, begins with a red macule or papule and then develops into an expanding erythematous rash with a bright red border known as erythema migrans (Fig. 14–30). The center of the rash can clear, indurate, blister, or become necrotic. Multiple annular lesions can develop.
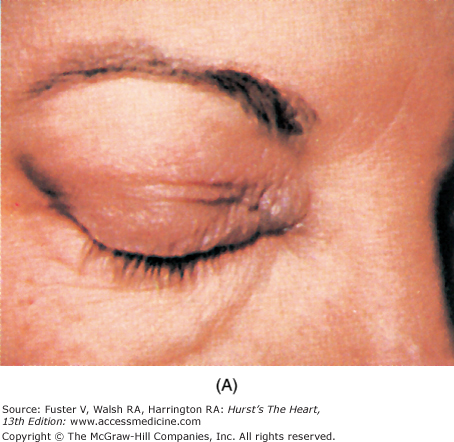
Diseases that cause myocardial fibrosis include dermatomyositis, Duchenne and Becker muscular dystrophy, myotonic muscular dystrophy, Kearns-Sayre syndrome, Friedreich ataxia, sarcoidosis, and scleroderma (see Chaps. 36 and 37). With dermatomyositis, a heliotropic discoloration is displayed on the upper eyelids (Fig. 14–31), and a scaly, erythematous papular rash—Gottron papules—can cover the knuckles, sparing the interphalangeal region. A waddling gait and pseudohypertrophic calves are characteristic of Duchenne muscular dystrophy, which can lead to fibrosis of the posterior LV. In myotonic dystrophy, drooping eyelids, cataracts, a receding hairline, and a mask-like expression are present. The Kearns-Sayre syndrome is a form of ocular muscular dystrophy in which external ophthalmoplegia, ptosis, and retinitis pigmentosa occur. Skin manifestations of sarcoidosis are common and include erythema nodosum; lupus pernio (a red or violet plaque formation with a predilection for the nasolabial folds, eyelids, and ears); and waxy, translucent papules found on the cheeks, periorbital area, ears, and elsewhere. Uveitis, bilateral parotid and lacrimal gland enlargement, and arthritis are other signs.
Infiltrative diseases of the myocardium include Wilson disease, Cori disease, Fabry disease, hemochromatosis, amyloidosis, glycogen storage disease, and sarcoidosis (Fig. 14–32). Wilson disease is an autosomal recessive disorder in which copper accumulates in tissues, including the myocardium. Arrhythmias, autonomic dysfunction, and cardiomyopathy have been reported. Kayser-Fleischer rings, usually golden-brown in color and circling the edge of the cornea, provide a major clue to the diagnosis.
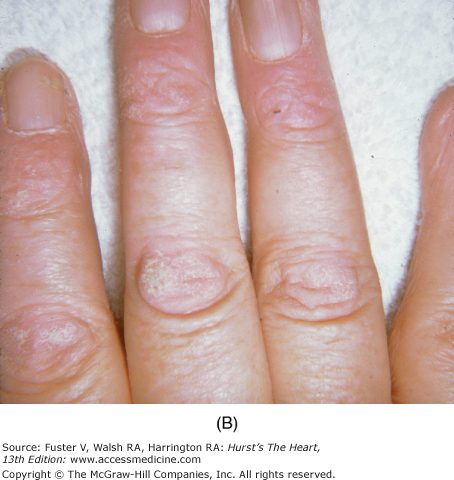
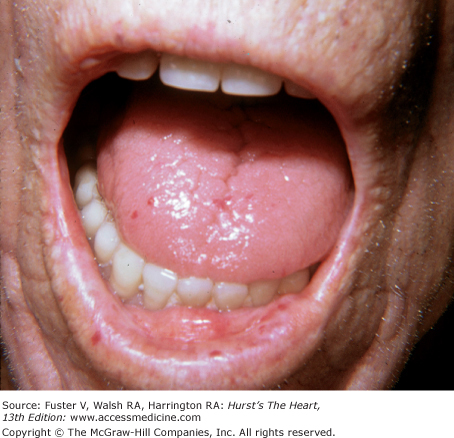
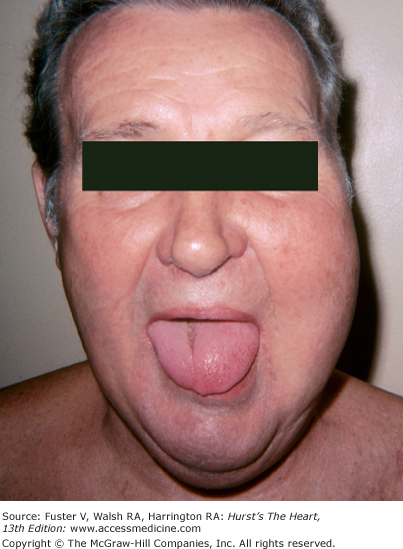
In hemochromatosis, the skin has a bronze or slate-gray coloration; myocardial infiltration with iron deposits can cause a dilated or rarely a restrictive cardiomyopathy associated with arrhythmias and heart failure. Macroglossia with indentations, waxy nodules of the skin and eyelids caused by fibrillar protein deposition, and “pinch purpura“ are clues to the diagnosis of amyloidosis (Fig. 14–33).
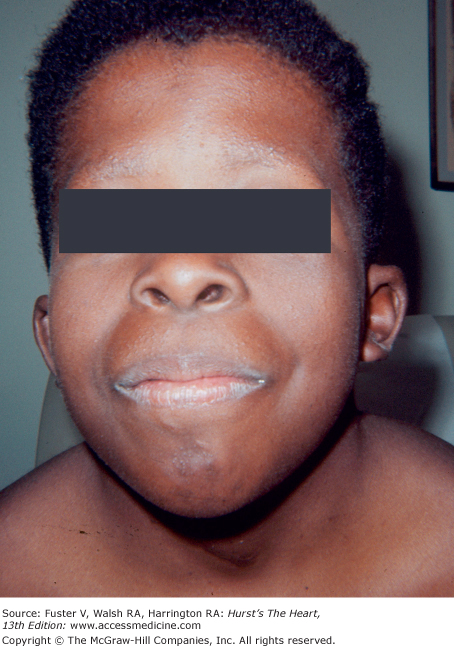
Pericarditis can be a result of Reiter syndrome, Whipple disease, Kawasaki disease, SLE, rheumatoid arthritis, rheumatic fever, sarcoidosis, scleroderma, dermatomyositis, hemochromatosis, Behçet syndrome, Degos disease, uremia, mulibrey nanism, polychondritis, hypothyroidism, or metastatic disease (see Chap. 90). The components of Behçet syndrome include erythema nodosum, superficial phlebitis, oral and genital ulcers, and iritis. Patients with Degos disease (malignant atrophic papulosis) present with painless, oval cutaneous lesions that have a white center and surrounding erythema. In this rapidly fatal disease, occlusive fibrosis of small- and medium-sized arteries produces pleuritis and pericarditis. In far-advanced renal disease, urochrome pigmentation of the skin and uremic frost can be cutaneous manifestations. Hypothyroidism, a cause of massive pericardial effusion, thickens the face and causes dry hair, puffy eyelids, and an enlarged tongue. Pericarditis is common in SLE but only rarely is the presenting manifestation.
Acquired causes of AV block or bundle-branch block include sarcoidosis, rheumatic fever, gout, Reiter syndrome, dermatomyositis, polychondritis, amyloidosis, Kawasaki disease, ankylosing spondylitis, SLE, and Lyme arthritis. In gout, uric acid crystals can form nodules affecting the conduction system. AV block can be an early cardiac manifestation of ankylosing spondylitis. Maternal lupus is an important cause of congenital complete AV block in newborns.
Inherited disorders associated with conduction defects include Fabry disease, Friedreich ataxia, Kearns-Sayre syndrome, multiple lentigines syndrome, muscular dystrophy, myotonic dystrophy, tuberous sclerosis, and Refsum disease. There is a high incidence of paroxysmal complete AV block with myotonic dystrophy, and pacing is often necessary.
Aortic aneurysms and dissection are frequent cardiovascular complications of Marfan and Ehlers-Danlos syndromes. Aneurysms of other vessels and arterial rupture can also occur. A progressive looseness of skin producing pendulous folds and droopy eyelids can be caused by cutis laxa, a generalized destruction of elastic tissue that can cause dilatation of the aorta or rupture of the pulmonary artery and aorta. Coronary aneurysms and occlusion—leading to arrhythmia, infarction, and sudden death—are late sequelae of Kawasaki disease, which can present in young adults.
Coronary atherosclerosis can be associated with hyperlipidemia, cerebrotendinous xanthomatoses, Werner syndrome, uremia, progeria, acromegaly, and diabetes mellitus. Hyperlipidemia can be suspected when xanthomas or arcus senilis are present. Xanthelasma is a skin condition usually involving lipid-laden plaques on the upper eyelid. When it occurs before age 50 years, there is a strong association with familial hyperlipidemia and premature CAD. Eruptive xanthomata are recognized as 1- to 4-mm papules with yellow centers surrounded by an erythematous halo. They often appear with a sudden outbreak of discrete lesions on the buttocks, back, thighs, and exterior surfaces of the knees and elbows. They indicate a very high level of triglycerides and are associated with hyperlipidemia, diabetes mellitus, pancreatitis, myxedema, and nephrotic syndrome. Tendon xanthomata are firm, painless nodules that thicken the exterior tendons of the hand, the Achilles tendons, and sometimes the tendons of the knees and elbows in patients with familial type II hyperlipidemia (Fig. 14–34).
In Werner syndrome, the skin is tightly stretched over the underlying bones. There is marked loss of subcutaneous tissue, and ulcerations occur over the legs. Severe coronary atherosclerosis often results in MI at an early age. Physical findings in diabetes mellitus can include tight skin and necrobiosis diabeticorum (Fig. 14–35), an atrophy of the skin of the lower extremities characterized by ovoid plaques with central telangiectasia and a violet, indurated perimeter. Progeria is a rare disorder in which the face is small and prematurely aged, the eyes bulge, and the nose is beaked. Severe atherosclerosis is a common cause of death in early life. A diagonal earlobe crease is curiously associated with coronary atherosclerosis and stroke and present in 60% of patients, including virtually all with onset before age 40 years (Fig. 14–36). Patients with homocystinuria resemble those with Marfan syndrome with long extremities, pectus carinatum, and kyphoscoliosis. Their cardiac disease differs from that of Marfan syndrome, with homocystinuria causing premature coronary disease. Pseudoxanthoma elasticum has been associated with fibrosis of the coronary artery and calcification of peripheral arteries. A glycosphingolipid is deposited in the arterial endothelium of patients with Fabry disease and can result in angina pectoris or MI. Patients with Hurler syndrome have mental retardation; a large, boat-shaped head; a broad nose; large lips; small, widely spaced teeth; and a large, protuberant tongue. Glycosaminoglycan deposition in the coronary arteries is present. Myocardial fibrosis caused by repeated spasm of the small coronary vessels has been postulated to be a result of scleroderma.
Vasculitis can be caused by SLE, rheumatoid arthritis, Behçet syndrome, Kawasaki disease, and polyarteritis. Cutaneous infarction, nodules, petechiae, livedo reticularis, gangrenous digits, MI, heart failure, and hypertension can be caused by polyarteritis (see Chap. 9). Cholesterol emboli attributable to extensive aortic atherosclerosis cause livedo reticularis and embolic or gangrenous changes in the toes simulating polyarteritis.
Arteriovenous shunts can be found in extensive skin disease, hereditary hemorrhagic telangiectasia, and the Klippel-Trenaunay-Weber syndrome. Kaposi sarcoma or exfoliative dermatitis caused by psoriasis can divert the blood supply through shunts in the skin to produce high-output cardiac failure. Telangiectasias of the fingertips (Fig. 14–37), face, palate, lips, and tongue, as well as pulmonary and hepatic arteriovenous fistulas, are components of hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome). The triad of anomalies that Klippel-Trenaunay-Weber syndrome comprises are vascular nevus, large varices, and bony or soft tissue hypertrophy (Fig. 14–38). Marked enlargement of a limb(s) and facial hemihypertrophy are features of this disorder, in which part or all of the deep venous system is absent and arteriovenous malformation is often present. Hemangiomas of the skin also can indicate multinodular hemangiomatosis of the liver, a cause of high-output heart failure in infancy.
Stenosis of large arteries can occur with supravalvular aortic stenosis, rubella syndrome, Turner syndrome, and neurofibromatosis. The face of a child with supravalvular aortic stenosis (Williams syndrome) is diagnostic (Fig. 14–39). The head is small, with an elf-like appearance; the cheeks are full and baggy; and the mouth and forehead are large. Curved lips and peg-shaped, widely spaced teeth are typical. Mental retardation is often present. Pulmonic artery branch stenosis is frequently present. Coarctation of the aorta is a common cardiac lesion in Turner syndrome, and neurofibromatosis has been associated with renal artery stenosis.
Multiple lentigines, cutaneous myxomas, myxoid fibroadenomas of the breast, and various endocrine abnormalities are features of an inherited disorder in which single or multiple cardiac myxomas occur. A susceptibility to atrial fibrillation and atrial flutter has been documented in patients who have fascioscapulohumeral muscular dystrophy. Sinus node dysfunction with atrial paralysis, elbow contractures, and humeroperoneal weakness are manifestations of Emery-Dreifuss muscular dystrophy.
Figure. 14–40 shows exophthalmus in a patient with atrial fibrillation and hyperthyroidism.
Single or multiple rhabdomyomas can develop within the myocardium and cause heart failure, valvular obstruction, or arrhythmias in patients with tuberous sclerosis. The diagnosis is suggested by the presence of yellow-brown angiofibromas (adenoma sebaceum) on the face (Fig. 14–41), subungual fibromas around the fingernail, café-au-lait spots, and subcutaneous nodules.
Measurement of Arterial Blood Pressure
For the noninvasive evaluation of arterial blood pressure, a pneumatic cuff with a mercury or aneroid manometer is the most frequently used instrument for assessing the status of the circulation and the interaction between the heart and arterial system. Blood pressure measurements above or below normal limits often provide important diagnostic information in patients with a variety of cardiac and noncardiac diseases. Accordingly, the blood pressure is best recorded by the physician during his or her initial physical examination.
See Chap. 69 for more information.
In 1733, Stephen Hales recorded the arterial pressures in animals by cannulation and use of a blood-filled glass column.17 Current methods for the direct and continuous measurement of arterial pressure use the electromanometer, a transducer that converts mechanical energy into an electric signal. The artery is cannulated with a saline-filled catheter or needle that mechanically couples the circulation to the arterial manometer. Pressures are recorded using atmospheric pressure as the zero reference level, and intravascular pressures are further referenced to the level of the heart by addition or subtraction of a gravitation factor. The gravitation factor is expressed as pgh, where p is the density of blood (in grams per milliliter), g is the acceleration caused by gravity (980 cm/s), and h is the transducer height (in centimeters) above or below the horizontal plane of the heart.
The strain-gauge manometer often is used for the precise and accurate measurement of the arterial pressure. However, error can originate in the catheter or coupling system when the properties of inertia, friction, and elasticity interact to produce damping of the frequency response. Either overdamping or underdamping can result in signal distortion. Nevertheless, the appropriate combination of an inelastic cardiac catheter and connecting tube filled with bubble-free fluid produces critical damping with the system response constant and adequate for the clinical recording of intravascular pressures.
Measurement errors also occur when an end-hole catheter is positioned axial to flow in a vessel and can become especially important during high arterial flow, when kinetic energy can exceed 10% of the total fluid energy. Also, pressure transients caused by catheter whip can falsely elevate the measured arterial pressure.
Miniature, self-flushing strain-gauge manometers attached directly to an intravascular catheter or needle reduce the problems related to transducer mounting and flushing. A more effective method for reducing measurement errors, however, is the use of intravascular electromanometers mounted on cardiac catheters or surgically implanted in the vascular wall.
The invention of the pneumatic cuff manometer (Riva-Rocci, 1896) and the subsequent discovery and use of the arterial sounds (Korotkoff, 1905) enabled indirect measurement of the arterial pressure.18 The mercury manometer is the gold standard; the aneroid manometer should be calibrated against the mercury manometer at least every 6 months. Semiautomatic electronic devices, if used, should be validated according to Association for the Advancement of Medical Instrumentation (AAMI) guidelines. The most commonly used noninvasive method is based on the auscultatory detection of low-pitched Korotkoff sounds over a peripheral artery at a point distal to cuff compression of the artery. McCutcheon and Rushmer defined two major components of these sounds: the initial transient (k–i) and the compression murmur (kc), which coincide with the opening tap and rumble sounds of Rodbard. The initial sound, k–i, occurs when cuff pressure reaches arterial pressure and likely results from abrupt arterial opening and vascular distension. The intensity of this initial sound depends on the slope of the pressure pulse and the level of the distal arterial pressure at the time of arterial opening, the sound being louder with vasodilatation and high-velocity flow and softer with arterial constriction or circulatory collapse. The initial transient is probably caused by oscillation of the arterial walls as the occluded segment is suddenly opened by systolic pressure, and the compression murmur is caused by a turbulent jet of flow distal to the partially compressed segment.
The Korotkoff sounds have been divided into five phases occurring in sequence as the occluding pressure declines (Table 14–4). To avoid error, the observer must be prepared to recognize two normal Korotkoff sound variations associated with blood pressure reading: (1) The auscultatory gap is a period of silence occurring during Korotkoff phases I and II. This disappearance of sound is temporary and is usually short, but the gap can occur over a 40-mm Hg measurement. (2) An absent Korotkoff phase V occurs when sounds are heard to 0. When this is the case, phase IV should be recorded along with phase V. In this case, phase IV is the best reference for diastolic pressure.
| Phase I: The pressure level at which the first faint, consistent tapping sounds are heard. The sounds gradually increase in intensity as the cuff is deflated. The first of at least two of these sounds is defined as the systolic pressure. |
| Phase II: The time during cuff deflation when a murmur of swishing sounds are heard. |
| Phase III: The period during which sounds are crisper and increase in intensity. |
| Phase IV: The time when a distinct, abrupt, muffling of sound (usually of a soft blowing quality) is heard. This is defined as the diastolic pressure in anyone in whom sounds continue to zero. |
| Phase V: The pressure level when the last regular blood pressure sound is heard and after which all sound disappears. This is defined as the diastolic pressure unless sounds are heard to zero. |
Proper technique is important for accurate measurement. The inflatable rubber bag within the compression cuff should have a width that is 20% greater than the limb diameter and a length adequate to encompass two-thirds of the limb. Before auscultation, the cuff is quickly inflated to a pressure 20 mm Hg above the systolic blood pressure, as indicated by obliteration of the radial pulse. The stethoscope is then applied lightly but firmly over the artery, and auscultatory pressure is determined by noting the onset (peak systole) and behavior of the Korotkoff sounds as the cuff is deflated at a rate of approximately 3 mm Hg/s. When the sounds disappear, the bag should be rapidly decompressed and 1 or 2 minutes allowed to pass before repeat determinations are made. The blood pressure should be taken with the subject upright as well as supine. Measurement of the blood pressure in both arms is recommended, especially in elderly patients. An American Heart Association hypertension primer recommends that the systolic pressure be recorded as the point at which the first tapping sounds occur for two consecutive beats (phase I) and that the diastolic pressure in adults be recorded as the point at which sounds become inaudible. In children and in adults with a hyperkinetic circulation, the diastolic pressure should be recorded as the point at which muffling of the sounds occurs (onset of phase IV). The arterial pressures at both the onset of muffling (phase IV) and the disappearance of sound (phase V) should be recorded. The mean blood pressure can be estimated by the addition of one-third the pulse pressure (systolic pressure minus diastolic pressure) to the diastolic pressure.
Patients with atrial fibrillation can have a significant beat-to-beat variation in their arterial pressure. Accordingly, the indirect blood pressure should be measured several times and the average noted.
This indirect method provides several potential sources of error because of improper equipment, inaccurate detection of the Korotkoff sounds, and observer techniques.19 The standard pneumatic cuff can often be unsatisfactory for blood pressure measurement in the arms or in the legs of very obese subjects. The arterial pressure can be underestimated if the cuff is deflated too rapidly or if inadequate inflation does not result in complete arterial occlusion. When the cuff is deflated too slowly or is immediately reinflated for multiple pressure determinations, the resulting venous congestion can elevate the diastolic pressure artificially and falsely decrease the systolic pressure by decreasing the intensity of phase I or phase II sounds to an inaudible level.
Studies correlating direct and indirect blood pressure measurements have, in general, shown a good correlation between indirect and direct measurements of blood pressure in the arm. The indirect method tends to underestimate systolic pressure by several millimeters of mercury, overestimate diastolic pressure by several millimeters of mercury when phase IV is used as an endpoint, and slightly underestimate diastolic pressure in normal individuals when phase V is taken as the endpoint.
Home blood pressure recordings using manual or automatic inflation and deflation of the cuff and detection of Korotkoff sounds by a microphone, stethoscope, or ultrasonic transducer are being used with increasing frequency for the ambulatory assessment of patients with hypertension. Although clinically useful, ambulatory blood pressure devices generally do not meet the standards for automated devices of the AAMI.
More recently, arterial tonometry has been used as a completely noninvasive method for monitoring the arterial pressure. This probe, with a micromanometer in its tip, operates on the principle of a piezo-resistive transducer of cantilever construction.20,21
Normal pressures have been defined on the basis of values included within two standard deviations of the mean of pressures obtained in a large population of apparently healthy individuals (see Chap. 67). The normal blood pressure range varies with age, gender, and race. In the United States, the pressure increases rapidly during the first few days of life and then increases gradually, with a slightly greater increment in systolic than in diastolic values, throughout life. The pressure tends to be higher in Western industrialized societies than in Asian, African, and technically undeveloped societies.
With increasing age and into senescence, the aorta undergoes progressive dilatation and elongation, with increasing stiffness of its walls.22 Resulting from this diminished vascular distensibility, there is an increase in systolic arterial pressure with less change in diastolic pressure.
The normal blood pressure limits for adults living in the United States are approximately 100 to 140 mm Hg systolic and 60 to 90 mm Hg diastolic. In an individual subject, however, baseline pressures above or below these levels do not define a pathologic state. The systolic arterial pressure rises slowly and progressively in most Americans between the ages of 20 and 60 years and more rapidly later, increasing by approximately 20 mm Hg between the ages of 60 and 80 years. Diastolic pressure usually rises very little after 45 years of age. Data from the Framingham Study and more recent studies (eg, MRFIT, SHEP, Syst-Eur) have shown a clear correlation between systolic blood pressure and cardiovascular events, a reduction in cardiovascular events with reduction of systolic blood pressure, or even a negative association between diastolic blood pressure and cardiovascular events.
In mildly to moderately hypertensive persons, the blood pressure casually recorded by a physician is significantly higher than the average value of a series of intermittent, indirect determinations or continuous direct recordings made during normal activity. To estimate basal blood pressure, measurements have been obtained during sleep, when the subject first awakens in the morning while still recumbent, or after several hours of reclining.
Factors contributing to variations in an individual’s blood pressure during daily activities include (1) body posture; (2) state of muscular, cerebral, or gastrointestinal activity; (3) emotional or painful stimuli; (4) environmental factors such as temperature and noise level; and (5) the use of tobacco, coffee, alcohol, and other drugs with direct or neurally mediated vasomotor properties.23 Twenty-four–hour pressures, obtained from normal and hypertensive subjects with an automatic recorder, have shown considerable variability with activity and emotional stimuli. The average diurnal pattern of blood pressure consists of an increase throughout the day and early evening and a significant, rapid decline to a low point during the early, deep stage of sleep. This is an important fact to remember in prescribing medications for patients with systemic hypertension (see Chap. 91).
With normal respiration, the peak systolic blood pressure is greater during expiration than during inspiration by as much as 10 mm Hg. An augmentation of this difference occurs in patients with pericardial tamponade (pulsus paradoxus; see Chap. 85) and during hyperventilation.
Isotonic exercise in both the supine and upright positions produces a moderate increase in blood pressure (systolic pressure greater than mean pressure, which is greater than diastolic pressure). Sustained isometric muscular contractions produce an abrupt increase in systolic, mean, and diastolic blood pressure that depends on the strength of the contraction.
An increase in arterial pulse pressure is commonly observed during routine blood pressure recordings. This usually is caused by an increase in stroke volume and ejection velocity, often with a decrease in peripheral resistance. Fever, anemia, hot weather, exercise, pregnancy, hyperthyroidism, or arteriovenous fistulas can produce this change. Several cardiac diseases, such as AR, patent ductus arteriosus, and truncus arteriosus, can also result in a widened pulse pressure. An increased pulse pressure caused by a large stroke volume can occur with complete heart block or marked sinus bradycardia.
Atherosclerosis of the large arteries often reduces arterial compliance and results in an elevated systolic pressure with a normal or even decreased stroke volume. The systolic hypertension of the elderly does not necessarily represent a change in arteriolar resistance. It is due to decreased aortic compliance. The increased pulse pressure associated with systemic arteriovenous fistulas is less common; a relative tachycardia can be the only clinical clue. Compression of a systemic arteriovenous fistula can produce a prompt slowing of the heart rate (Branham sign).
A narrow pulse pressure is uncommon in normal subjects but can result from an increased peripheral resistance (increased circulating catecholamines in heart failure), decreased stroke volume (severe as in arteriosclerosis or aortic stenosis), or markedly decreased intravascular volume (diabetic ketoacidosis).
The diagnostic importance of blood pressure differences between the right and left arms can occur as a result of supravalvular AS as well as the choanal effect in children and the subclavian steal syndrome in adults.24 Most patients with the former have greater than 20 mm Hg higher blood pressure in the right arm. The subclavian steal syndrome, often accompanied by symptoms of cerebrovascular insufficiency, usually results in a pronounced lowering or absence of brachial artery pressure in the ipsilateral extremity. There is diminished vertebral blood flow to the brain because flow is diverted to the involved subclavian artery distal to the stenosis.
A progressive increase in systolic pressure normally occurs as the point of measurement is moved peripherally from the central aorta (Fig. 14–42); the increment is similar in the large arteries of the upper arm and the thigh. Direct recordings of femoral and brachial arterial pressures (systolic, diastolic, and mean) in adults and children and indirect measurement of popliteal and brachial artery pressures have demonstrated that mean pressures are equal at these sites. A difference in arm and leg pressures can occur because of coarctation of the aorta or acquired disease, such as peripheral arteriosclerosis, aortic dissection, aortic arch syndrome, or the subclavian steal syndrome.
Figure 14–42
Micromanometer and catheter tip flow velocity as change in contour of pressure waves (above) and flow waves (below) between the ascending aorta and the saphenous artery. Reprinted from Vlachopoulos C, O’ Rourke MF. The arterial pulse. CurrProbl Cardiol. 2000;25:296-346, with permission from Elsevier.
Pulsus alternans can be detected by palpating a peripheral artery, preferably the femoral artery, when the heart rhythm is normal. The sphygmomanometer can be used to measure accurately the beat- to-beat variation in pressure that characterizes pulsus alternans.
Pulsus alternans occurs in patients with severe heart disease who exhibit impaired LV contraction. It can also occur for several beats after supraventricular tachycardia in normal persons or when the respiratory rate is half the pulse rate.
A normal person can exhibit a 10–mm Hg decrease in systolic blood pressure during normal inspiration. A greater decline can be identified in patients with acute cardiac tamponade, constrictive pericarditis, severe obstructive lung disease, and restrictive cardiomyopathy.
Pulsus paradoxus is best detected by inflating the blood pressure cuff above systolic pressure and then slowly releasing it. As the cuff pressure is gradually reduced, the blood pressure sounds become audible during expiration. The difference in pressure between the first audible sound heard on expiration and the pressure level at which the sounds are heard during all phases of respiration gives a measurement of magnitude of pulsus paradoxus. The mechanism of pulsus paradoxus is discussed in Chap. 85.
The Arterial Pulse
The arterial pulse, similar to any periodic fluctuation that is caused by the heart, occurs at the same frequency as the heartbeat. Ejection of blood with every cardiac contraction is converted to flow, pressure, and dimension pulsations in arteries throughout the body. Although the term pulse refers to any such pulsation, the arterial pulse perceived by a clinician is the pressure pulse in a large, accessible artery. Palpation of the arterial pulse is a basic and important element of the physical examination.25
Pressure and blood flow in the ascending aorta result from the interaction between the heart and arterial system. When LV pressure exceeds the aortic pressure, it becomes the driving force for the movement of blood into the ascending aorta. This driving force depends on the intrinsic contractility of ventricular muscle, the size and shape of the LV, and the heart rate. It is opposed by several forces that impede the development of flow and are interrelated in a complex manner. Three major determinants of arterial impedance are resistance, inertia, and compliance.
Resistance is related to blood viscosity and the geometry of the vasculature; it opposes flow and is unaffected by changes in heart rate. Inertia, which is related to the mass of the column of blood, opposes the rate of change of arterial blood flow (ie, acceleration) and depends on the heart rate. Compliance is related to the distensibility of the vascular walls, opposes changes in arterial blood volume, and depends on the heart rate. The heart rate dependency of inertia and compliance introduces phase shifts between instantaneous pressure and flow in a pulsatile system. Inertia and compliance are important determinants of the character of ventricular ejection, especially in early systole, when flows and pressures change rapidly.
The arterial pulse wave begins with aortic valve opening and the onset of LV ejection. Aortic pressure increases rapidly in early systole because the LV stroke volume enters the aorta faster than it flows to distal sites. The rapid-rising portion of the arterial pressure curve is often termed the anacrotic limb (from the Greek meaning upbeat). In experimental animals and in humans, peak proximal aortic flow velocity occurs slightly earlier than peak pressure. After its peak, aortic pressure declines as LV ejection slows, and peripheral blood flow continues. During isovolumic relaxation, a transient reversal of flow from the central arteries toward the ventricle just before aortic valve closure is associated with an incisura on the descending limb of the aortic pressure pulse. The subsequent smaller, secondary positive wave has been attributed to the elastic recoil of the aorta and aortic valve but is partially caused by reflected waves from more distal arteries. Subsequently, aortic pressure decreases again as further runoff in the peripheral circulation occurs in diastole.
The proximal aortic pulse pressure is directly proportional to the ratio of stroke volume to arterial distensibility, but multiple factors influence this complex relationship.26 Arterial distensibility diminishes as the distending arterial pressure increases. Accordingly, the pulse pressure for a constant stroke volume will be larger if the mean blood pressure is elevated. Also, arterial distensibility varies inversely with the rate of increase of intraluminal pressure. When the systolic ejection rate increases, the stiffer arterial wall results in a greater pulse pressure.
Pulsatile changes in arterial diameter are virtually identical to the pressure pulse, with minor differences explained in terms of nonlinear elasticity and viscosity of the arterial wall. The pulse waveform recorded at any site of the arterial tree is the sum of a forward waveform and a backward-traveling one that is the echo of the incident wave reflected at peripheral sites. Wave reflection is an important determinant of LV load and CBF. A reflected wave occurring at systole increases systolic pressure and thereby increases ventricular afterload. In contrast, occurrence of the reflected wave at diastole is highly desirable because augmentation of pressure during diastole aids coronary perfusion.
Conventionally, the pulse is described in the time domain, where it is considered as a change in arterial pressure with time. An alternative, quantitative approach is to analyze the pulse in the frequency domain. Pulse is conceived as a composite wave that can be resolved into component harmonics similar to a musical wave. Impedance is the measure of the opposition to flow presented by a system and can be measured when harmonic analysis is used to relate frequency components of pressure and flow pulses.26
Usually there is a linear relation between pressure and flow at the same point in an artery and between pressures at different points in the arterial system. From impedance curves, it is possible to identify the factors responsible for the relationship between the pulsatile pressure and flow. The peripheral arterial pressure wave recorded is the summation of the incident (initial) and reflected waves. The systemic circulation has been represented by a simple asymmetric T-tube model that emphasizes the importance of wave reflection at two arteriolar reflecting sites in the upper and lower parts of the body. An important patient study indicates major reflection sites at the aortic level of the renal arteries and at a point distal to the terminal abdominal aorta bifurcation.
As the normal aortic pulse wave is transmitted peripherally, significant changes in its contour occur caused by (1) distortion and damping of pulse wave components; (2) different rates of transmission of various components; (3) distortion or exaggeration by reflected, resonant, or standing waves; (4) conversion of kinetic energy into hydrostatic or potential energy; (5) differences in distensibility and caliber of the arteries; and (6) changes in the vessel wall caused by age, disease, or both.
The arterial pressure pulse enters the proximal aorta and travels distally at a velocity many times faster than that of maximum blood flow. The pressure wave is accompanied by a traveling wave distending the arterial wall with the pulse wave velocity increasing as arterial wall distensibility diminishes.
The pulse wave arrives progressively later at more peripheral sites when timed from the QRS complex on the ECG. Representative time delays from the central aorta are as follows: carotid, 30 ms; brachial, 60 ms; radial, 80 ms; and femoral, 75 ms.
The arterial pulse wave undergoes a progressive change in shape during its transmission distally (see Fig. 14–42). The pulse pressure and systolic amplitude increase, and the ascending limb of the pulse wave becomes steeper. The incisura of the central aorta pulse is gradually replaced by a smoother, somewhat later dicrotic notch that occurs at lower pressure levels. The dicrotic notch and the following positive secondary or dicrotic wave probably result from the summation of the forward pulse wave and reflected waves from the peripheral vessels.
The ankle-brachial index (ABI) is the ratio of the systolic blood pressure at the ankle divided by the higher of the two arm systolic blood pressures.15 It reflects the degree of lower extremity arterial occlusive disease, which is manifest by reduced blood pressure distal to stenotic lesions. Either the posterior tibial or dorsalis pedis artery pressures can be used. It is important to note that each equally reflects the status of the aortoiliac and femoropopliteal segments but different tibial arteries; therefore, the resulting ABIs can differ. An arm systolic pressure of 120 mm Hg and an ankle systolic pressure of 60 mm Hg yields an ABI of 0.5 (60/120 mm Hg). The ABI is inversely related to disease severity. A resting ABI below 0.9 is considered abnormal. Lower values correspond to progressively more severe occlusive peripheral arterial disease (PAD) and disabling claudication. An ABI below 0.3 is consistent with critical ischemia, rest pain, and tissue loss. This concept has been in widespread clinical use for several decades.
A reduced ABI is generally equated with atherosclerotic lower extremity PAD. However, other forms of arterial disease, such as giant cell arteritis, dissection, and emboli, can cause lower extremity arterial occlusions or stenoses, thus yielding an abnormal ABI. False-negative results can be encountered in diabetics with calcified noncompressible tibial and pedal vessels. Because arm pressure is used as the denominator in calculating ABI, stenotic disease of the brachiocephalic, subclavian, axillary, or brachial arteries can lower arm pressure and give an ABI that does not reflect the lower extremity arterial occlusive disease. There are patients with increasing ABIs who were thought to have improving lower extremity circulation but who were in fact experiencing worsening upper extremity arterial occlusive disease. For screening purposes, ABI is usually measured only at rest. However, a resting ABI above 0.9 does not exclude significant PAD. More proximal stenoses, particularly at the aortoiliac level, may not be severe enough to reduce ankle pressure at rest but can cause a precipitous decrease in ABI after exercise.
Because atherosclerosis is a systemic process, a patient with PAD and an abnormal ABI has an increased risk of disease in other vascular territories, including the coronary and cerebral circulation, putting him or her at risk for major adverse cardiovascular events.
There is a highly significant trend toward increasing relative risk of history of manifest cardiovascular disease with decreasing ABIs. In the Cardiovascular Health Study, the 7.4% of participants with an ABI below 0.8 were more than twice as likely to have a history of prior coronary event, cerebrovascular event, or congestive heart failure than were those with a normal ABI (P <.01).
All major arterial pulses should be examined bilaterally for both patency and waveform characteristics. The thickness and hardness of the arterial walls often can be assessed by rolling the vessel against underlying tissue. A pulse in the foot should not be considered absent unless examined with the foot in a dependent position. Otherwise, the arterial pulses usually are examined with the patient supine and with the trunk of the body slightly elevated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree