The Heart and Pregnancy
Kenneth L. Baughman
Overview
Pregnancy normally induces significant physiologic adaptation in the cardiovascular system, including increases in heart rate, left ventricular size, stroke volume, and left ventricular mass. Systemic vascular resistance decreases during pregnancy. The maximal increase in hemodynamic burden for the pregnant woman is achieved at the end of the second trimester. Uterine contractions and the sympathetic discharge associated with delivery further increase cardiovascular demands, with the greatest increase in cardiac output achieved in the final stages of delivery. These alterations are resolved approximately 6 weeks after delivery. Moderate aerobic exercise during pregnancy is safe, increasing maximal aerobic power and the capacity for sustained submaximal exercise, as well as preserving aerobic capacity in late gestation have been demonstrated.
Hypertension complicates 10% of pregnancies, is rarely due to secondary causes, and is classified as chronic hypertension, gestational hypertension, or preeclampsia. Preeclampsia, the most worrisome of these disorders, involves hypertension, proteinuria, edema, and possibly coagulopathy and liver dysfunction. If preeclampsia is severe, the condition may lead to eclampsia, a seizure disorder associated with high morbidity and mortality. The HELLP syndrome (hemolysis, elevated liver function tests, and low platelet levels) is a preeclampsia variant that has the same potential for malignant degeneration to eclampsia.
Peripartum cardiomyopathy is the presence of a new cardiomyopathy, without any other cause of congestive heart failure or preexisting heart muscle disorder, that appears in the final month of the pregnancy or in the first 5 months postpartum. Older patients, patients experiencing multibirth pregnancies, patients with toxemia, and patients carrying a first child are somewhat more likely to experience this condition. Most patients with this condition present within 1 to 2 months of delivery. Myocarditis is frequently found in patients who undergo endomyocardial biopsy early after presentation.
All forms of chronic anticoagulation may result in bleeding between the uterus and placenta and subsequent pregnancy loss. Heparin may cause osteoporosis if it is administered at a high dose for long periods, and warfarin (Coumadin) may be associated with an embryopathy or central nervous system abnormalities. Patients who require anticoagulants should receive heparin or low-molecular-weight heparin in the first trimester and in the terminal stages of pregnancy. Coumadin can usually be safely administered through the remainder of pregnancy until just before delivery.
Bioprosthetic heart valves may degenerate during or after pregnancy and require replacement. Pregnant patients who have mechanical prostheses have a lower live birth rate and higher incidence of thromboembolic complications.
Myocardial infarctions associated with pregnancy are unusual; however, pregnancy is associated with an increased incidence of vasospasm and coronary dissection. Pregnant patients are also predisposed to aortic dissection, particularly if they have aortic disease, including Marfan syndrome or Takayasu aortitis.
Patients who have congenital heart disease, even if it is corrected, must be screened carefully before they become pregnant to ensure that their pulmonary vascular resistance, ventricular function, valvular insufficiency, prosthetic devices, and aortopathy will be able to tolerate the cardiovascular strains of pregnancy.
Tocolytic therapy consists primarily of the use of β-sympathomimetic agents to decrease uterine contraction and allow fetal lung maturation. Approximately 5% of exposed mothers develop pulmonary edema, which appears to be a capillary leak syndrome.
Because antiarrhythmic agents pose a significant risk to the patient and fetus, they must be chosen carefully.
Glossary
Bioprosthesis
An artificial valve made of biologic material, including either homograft (human) or heterograft (pig) tissue.
Embryopathy
Damage to the embryo, usually as a result of maternal exposure, typically in the first trimester of pregnancy.
HELLP syndrome
Hemolysis, elevated liver function tests, and low platelet levels in pregnant patients. Considered to be a variant of preeclampsia.
Myocarditis
Inflammation of the myocardium, characterized by a significant inflammatory infiltrate associated with myocyte necrosis.
Peripartum cardiomyopathy
Cardiomyopathy appearing in the final month of pregnancy or the first 5 months postpartum, with no preexisting heart muscle disorder and absence of any other cause of congestive heart failure.
Preeclampsia
The appearance of hypertension, proteinuria, and edema in a patient of more than 20 weeks’ gestation.
Teratogenic risk
Risk of an induced fetal abnormality, usually caused by maternal exposure in the first trimester of pregnancy.
Tocolytic therapy
Sympathomimetic therapy used to decrease uterine contractions; usually initiated to prolong gestation and increase fetal lung maturation.
Introduction
Cardiologists and internists are increasingly being consulted in the management of cardiovascular complications associated with pregnancy. A greater number of women with known or potential cardiovascular disease are becoming pregnant, and cardiovascular complications in pregnant women are increasingly recognized.
This chapter discusses the normal morphologic and physiologic changes that occur during pregnancy, as well as the pathophysiology and management of hypertension associated with pregnancy and peripartum cardiomyopathy. Treatment of pregnant patients who have congenital heart disease or artificial heart valves and of those who require anticoagulation during pregnancy is outlined. Detection and management of coronary artery disease and coronary dissection, as well as the use of a cardiopulmonary bypass during pregnancy, are reviewed. Avoidance of fetal risk factors during pregnancy, including toxic drinking water, is addressed, as are the potential cardiac dangers of the use of tocolytic therapy to retard uterine contraction. Finally, management of both supraventricular and ventricular tachycardia in pregnancy is reviewed.
Normal Physiologic and Morphologic Changes in Pregnancy
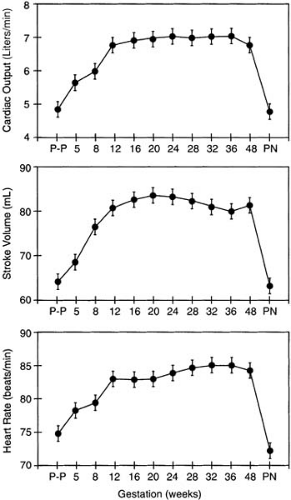
Striking adaptations occur in the maternal cardiovascular system in response to pregnancy. An appreciation of these changes is mandatory to allow appropriate treatment of pregnant patients who have cardiovascular disease. Hunter and Robson (1) summarized hemodynamic and structural changes in maternal subjects based on echocardiographic studies. Longitudinal studies of cardiac output began before conception and continued into the postnatal period. Cardiac output increases as early as 5 weeks after the last menstrual period and rises to 45% above baseline at 24 weeks’ gestation. Increased cardiac output is achieved by an increase in heart rate, which rises progressively until 32 weeks’ gestation, and in stroke volume, which begins to increase by 8 weeks and peaks as early as 20 weeks (Fig. 31.1). Twin pregnancies result in an additional 15% rise in cardiac output in mothers. The cardiovascular system is taxed further by stage 1 of labor, which is associated with an additional 12% increase in demand for cardiac output. This demand increases to a mean of 34% above the already increased baseline value as labor progresses to its final stages. After delivery stroke volume decreases by 2 weeks postpartum. Heart rate remains elevated for 2 days postpartum and returns to baseline by 10 days after delivery. Cardiac output similarly decreases from pregnancy levels to normal levels between 24 hours and 10 days postpartum. The magnitude of these echocardiographic changes was confirmed by Mabie et al. (2) and Vered et al. (3), who in addition documented an increase in left ventricular mass during pregnancy.
The structural changes noted by echocardiography are secondary to the alterations in intravascular volume and neurohumoral stimulation that are associated with pregnancy. Plasma volume increases approximately 40% and red blood
cell volume 30% above baseline prepregnancy levels, resulting in mild relative anemia (4). Estrogen-mediated stimulation of the renin–angiotensin axis results in increased renal tubular absorption of sodium and an increase in total body salt and water, contributing to the increased plasma volume. Systemic vascular resistance and diastolic blood pressure decrease as a result of changes in aortic compliance and arterial venous shunting in the uterus.
cell volume 30% above baseline prepregnancy levels, resulting in mild relative anemia (4). Estrogen-mediated stimulation of the renin–angiotensin axis results in increased renal tubular absorption of sodium and an increase in total body salt and water, contributing to the increased plasma volume. Systemic vascular resistance and diastolic blood pressure decrease as a result of changes in aortic compliance and arterial venous shunting in the uterus.
TABLE 31.1 Physical Findings during Normal Pregnancy | |
|---|---|
|
The normal physiologic changes associated with pregnancy result in alterations in the physical examination of the pregnant patient (Table 31.1). Because of the increase in total body salt and water, as well as plasma volume, the central venous pressure may be slightly increased and the pregnant patient may have 1 to 2+ lower-extremity edema. As pregnancy progresses, increased uterus size forces both diaphragms upward, which may decrease pulmonary vital capacity and total lung volume. The previously documented changes in left ventricular size and mass result in a mildly displaced and diffuse point of maximal impulse of the left ventricle and increased valve closure sounds. Because of the insulation of the atrioventricular sounds by the abdomen, only the aortic and pulmonic closure sounds are characteristically louder on examination. Increased flow through the pulmonic valve causes a “functional” early systolic murmur and slight widening of the physiologic variation of closure of the pulmonic component of the second heart sound. Ventricular gallop rhythms and diastolic murmurs are unexpected. Occasionally, a normal physiologic diastolic murmur can be heard at the left sternal border in pregnant patients. This physiologic murmur is usually generated by increased diastolic flow through the internal mammary artery. This “mammary soufflé” may persist in lactating mothers, even after delivery. The expected decrease in aortic diastolic pressure during pregnancy that is associated with the decreased systemic vascular resistance results in a widened pulse pressure and pulsatile fingertips, warm hands, and occasional Quincke sign in the extremity nail beds. These findings are also suggestive of aortic regurgitation that may be confused by the diastolic murmur of the mammary soufflé at the sternal border. Echocardiography may be required to distinguish the presence or absence of aortic valve disease.
The physiologic changes associated with pregnancy may also alter the results of noninvasive evaluations of the heart (Table 31.2). The increase in size and left ventricular mass seen by echocardiography has already been described. The electrocardiogram may also be altered. Upward movement of the diaphragm results in a leftward shift of the electrical axis of ventricular depolarization, and increased ventricular mass may result in increased ventricular voltage. Heart rate may be increased, particularly late in pregnancy, and atrial as well as ventricular arrhythmias may increase in frequency in patients predisposed to such events. Chest radiographic studies reveal a mild increase in cardiac size, a horizontal shift of the heart that increases with the duration of pregnancy, and fullness of the left cardiac border and pulmonary vascular supply (5).
TABLE 31.2 Changes in Noninvasive Test Results That Occur during Pregnancy | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Exercise can usually be maintained throughout pregnancy. Potential risks of exercise include hyperthermia-induced neural tube defects and decreased placental/fetal oxygen and substrate, which theoretically could alter fetal growth. Aerobic exercise to 69% maximal heart rate in pregnant patients does not alter core temperature, and oxygen saturation does not fall below 95% (6,7). Maternal exercise does increase fetal heart rate and fetal heart rate variability in the 20-minute postexercise interval (8,9). Potential exercise benefits include maintenance of aerobic capacity, enhanced cardiopulmonary reserve, and improved psychologic well-being and may help to prevent low back pain and gestational glucose intolerance (10,11). The increase in maternal resting heart rate and decreased maximal heart rate in pregnancy narrow the window of exercise heart rate response. Although there are no controlled trials, exercise is considered safe in pregnancy if there are no contraindications, nonballistic aerobic exercise is chosen, and excess ambient temperature and extremes of exertion are avoided (12,13,14).
Radiation and Pregnancy
X-rays produce direct and indirect damage to cells. Direct damage results from the effect of the high-energy radiation beam on cellular and molecular structure. Indirect damage is the result of ionized water and free radical generation. The strength and potential damage of x-rays are measured by the amount of ionic charge created per unit mass of air. One R (roentgen) creates more than 2 billion ion pairs per cubic centimeter of exposures to air. The absorbed dose of radiation is measured in rad, calculated by the energy imparted per unit mass of tissue. One rad is equivalent to the deposition of .01 J of energy per kilogram of tissue. One R of radiation usually produces 1 rad in tissue. Roentgen-equivalent-man (REM) is a measure of absorbed radiation modified by local tissue characteristics, which better delineates the risk of radiation damage to a given organ (15).
Radiation is harmful to all living tissue and particularly to the conceptus. Increasing radiation exposure is associated with a range of tissue defects, beginning with isolated cellular damage and progressing to growth impairment, structural deformity, neoplasia, and gonadal damage. Environmental radiation results in 50 to 100 mREM of exposure during the 9 months of childbearing. The amount of radiation considered
to be “safe” is unclear; however, virtually no data have demonstrated significant fetal damage after exposure to less than 1 rad (16).
to be “safe” is unclear; however, virtually no data have demonstrated significant fetal damage after exposure to less than 1 rad (16).
TABLE 31.3 Percentage Likelihood That Childhood Cancer Will Not Develop after Prenatal Irradiation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Several features alter the amount of radiation exposure that occurs during radiologic procedures, including the nature of the radiation source, the equipment used, the size of the patient, the depth of the uterus and conceptus, and the distance from the area being investigated, as well as the extent of the radiologic study. The dose of radiation received by the uterus may vary 50-fold depending on these features and whether appropriate uterus shielding is performed.
The increased risk of development of a subsequent malignancy by children receiving radiation in utero was reported by the Childhood Cancer Research Group in 1956 and confirmed in 1975 (15). Bithell and Stewart (16) demonstrated a relative risk of 1.47 for the development of subsequent malignancy in children whose mothers underwent radiographs during pregnancy. Furthermore, the relative risk increased progressively from 1.26 for one film exposure to 2.32 for five or more x-rays, indicating a dose response. A first-trimester exposure resulted in a relative risk of malignancy of 8.95, falling to 1.25 and 1.41 in the second and third trimesters, respectively. The risk of childhood cancer from radiation exposure is displayed in Table 31.3 (16).
The risk of radiation must be evaluated relative to the benefit to the mother. It is imperative that the clinician define (a) the dose of radiation to the conceptus (Tables 31.4 and 31.5), (b) the age and development of the conceptus, (c) the radiation risk from the radiation source, (d) the risk associated with delaying or not performing the test, and (e) the potential for alternative means of answering the question with nonradiation studies (17,18).
TABLE 31.4 Average Dose (mrad) to Uterus Based on 1,000-mREM Exposure | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Hypertension during Pregnancy
High blood pressure may complicate as many as 10% of all pregnancies and remains a major cause of maternal and fetal morbidity and mortality (19,20). In normal pregnancy, diastolic blood pressure falls, often by as much as 10 mm Hg in the first and second trimesters as a result of decreased systemic vascular resistance. Blood pressure then increases gradually at or near term and may transiently rise higher than nonpregnant values in the immediate puerperium. Rarely is high blood pressure in pregnancy the result of secondary causes. The categories for high blood pressure in pregnancy are (a) chronic hypertension, (b) gestational hypertension, and (c) preeclampsia, with or without preexisting high blood pressure.
Chronic high blood pressure is categorized as preexistent hypertension or a blood pressure of at least 140/90 mm Hg before 20 weeks’ gestation. If the diastolic blood pressure is 110 mm Hg or greater, severe hypertension is diagnosed. Pregnant women with a diastolic blood pressure higher than 75 mm Hg in the second trimester and 95 mm Hg in the third trimester should be observed carefully (21). The maternal and fetal outcomes are good unless preeclampsia or abruptio placentae complicates the final stages of gestation. Clear evidence exists (22) that treatment of diastolic blood pressure of greater than 110 mm Hg during pregnancy lowers the risk of stroke and cardiovascular complications, although the benefit of lowering blood pressure in mild chronic hypertension during pregnancy is less clearly defined.
Gestational hypertension is defined as asymptomatic blood pressure elevation after 20 weeks’ gestation. Unless this elevation of pressure reflects early preeclampsia or unrecognized chronic hypertension, the maternal and fetal outcomes are usually very good, even without treatment.
Preeclampsia is the most feared hypertensive disorder of pregnancy and may lead to life-threatening eclampsia, a convulsive disorder. Preeclampsia is characterized by hypertension, proteinuria (levels >300 mg in 24 hours), and edema but may include coagulopathy and altered liver function in pregnant patients after 20 weeks’ gestation (Table 31.6). Preeclampsia may be mild or severe, depending on the degree of blood pressure elevation and proteinuria. In preeclampsia, plasma volume and cardiac output fail to rise, and systemic resistance does not fall. There is an initial alteration in placental perfusion and angiogenic growth factors that leads to generalized damage to the endothelium (23) and release of placental debris in the circulation (24). This appears to be related to failure of the uterine spiral arteries to develop, preventing the anticipated arteriovenous shunting. Some researchers believe that the primary abnormality causing preeclampsia is the result of prostaglandin
deficiency; endothelial cell dysfunction; immune, genetic, or sodium/calcium membrane disorders; increase in sympathetic nervous system activity (25); and increased levels of platelet-activating factor (26), plasma levels of prostaglandin isoforms (27), and angiotensin-1 receptor autoantibodies (28). These observations may have ramifications for treatment. There may be a genetic susceptibility for preeclampsia/eclampsia, and preliminary data indicate chromosome 2 as the likely location (29). The maternal risks of preeclampsia include cerebral hemorrhage, pulmonary edema, disseminated intravascular coagulopathy, liver failure, renal failure, convulsions, and death. These risks are correlated with the severity of the complications and level of gestation at the onset of the condition. The fetus may suffer hypoxemia, acidosis, growth retardation, and death.
deficiency; endothelial cell dysfunction; immune, genetic, or sodium/calcium membrane disorders; increase in sympathetic nervous system activity (25); and increased levels of platelet-activating factor (26), plasma levels of prostaglandin isoforms (27), and angiotensin-1 receptor autoantibodies (28). These observations may have ramifications for treatment. There may be a genetic susceptibility for preeclampsia/eclampsia, and preliminary data indicate chromosome 2 as the likely location (29). The maternal risks of preeclampsia include cerebral hemorrhage, pulmonary edema, disseminated intravascular coagulopathy, liver failure, renal failure, convulsions, and death. These risks are correlated with the severity of the complications and level of gestation at the onset of the condition. The fetus may suffer hypoxemia, acidosis, growth retardation, and death.
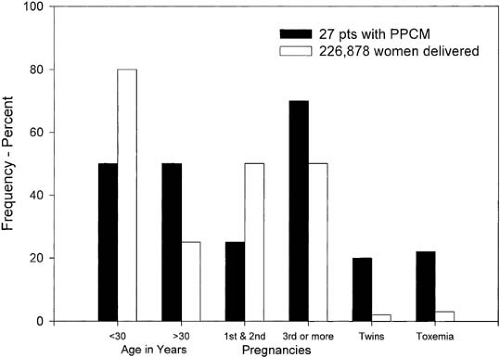 FIGURE 31.2. Risk factors for the development of peripartum cardiomyopathy (PPCM). pts, patients. (From Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation 1971;44:964–968. ) |
TABLE 31.5 Conceptus Dose Estimates from Radionuclide Exposure | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Mild preeclampsia may be managed at home; however, development of progressive symptoms such as headache, visual disturbances, and abdominal pain requires hospitalization (Table 31.7). The use of antihypertensive or anticonvulsant therapy to treat mild preeclampsia remains questionable. Treatment of severe preeclampsia, on the other hand, may require termination of the pregnancy, regardless of fetal viability, if the mother’s life is in jeopardy. Two recent large prospective, randomized trails have demonstrated the superiority of magnesium sulfate over phenytoin (anticonvulsant) and namodipine (calcium channel blocker with cerebral vasodilating properties) in prevention of eclampsia in women with severe preeclampsia (30,31,32). Antihypertensive therapy should be initiated with hydralazine, labetalol, or nifedipine to lower mean blood pressure and diastolic blood pressure to 126 and 90 mm Hg, respectively. Because these patients are already volume depleted, use of diuretics should be avoided. Intravenous magnesium should be given during labor and delivery and for 24 hours postpartum for patients who have severe preeclampsia to avoid convulsions.
TABLE 31.6 Typical Findings in Patients with Preeclampsia | |
|---|---|
|
There is no known mechanism for avoidance of preeclampsia, although sodium chloride restriction, diuretics, low-dose aspirin, and increased dietary calcium have been attempted.
A variant of preeclampsia, the HELLP syndrome, is characterized by minimal or no elevation of blood pressure, mild decreases in platelet levels, hemolysis, mild liver function abnormalities, and absence of renal impairment at the onset (20,22). This syndrome may progress rapidly, resulting in hemolysis, thrombocytopenia, and liver failure. Recognition of this syndrome prepartum should prompt immediate delivery. O’Brien and coworkers (33) suggested that corticosteroids may be of some benefit in this unusual disorder; however, a subsequent randomized, placebo-controlled trial comparing dexamethazone to saline control did not reduce disease severity or duration (34). Both the HELLP syndrome and preeclampsia may occur up to 10 days postpartum.
TABLE 31.7 Ominous Signs and Symptoms in Women with Preeclampsia | ||
|---|---|---|
|
Long-term follow-up (24 to 36 years postpartum) of patients with preeclampsia demonstrated an increased risk for death (2.1, 95% confidence interval [CI] 1.8 to 2.5) with deaths from cardiovascular events contributing most strongly to this increase (35).
Treatment of Hypertension during Pregnancy
Medications commonly used to treat chronic, gestational, and preeclamptic hypertension are listed in Tables 31.8 and 31.9. Appropriate medical management of pregnancy-related hypertension depends on an understanding of the pathophysiology outlined in the previous sections. Some changes are unique to alterations in kidney handling of sodium and water.
The mean increase in total body water and sodium during pregnancy is 6 to 8 liters and 500 to 900 mEq, respectively, approximately one-half of which is extracellular (36). Many factors alter sodium excretion in pregnancy; despite a 50% increase in the glomerular filtration rate, elevated levels of progesterone and aldosterone result in a 40% increase in plasma volume. The result is that more than 80% of all healthy pregnant women develop physiologic-dependent or generalized edema. Edema may be a normal physiologic response; hypertension and preeclampsia are not. Despite the increase in interstitial volume that occurs with preeclampsia, the intraventricular volume is reduced, and oliguria and hemoconcentration are typically present. There is no evidence that the use of sodium restriction or diuretics prevents preeclampsia or other hypertensive complications of pregnancy, and use of such agents may cause additional volume contraction, alkalosis, electrolyte abnormalities, pancreatitis, and bleeding or hyponatremia in the neonate (21,36). Use of diuretics in women with preeclampsia should be avoided, and these agents are of limited benefit for treatment of pregnancy-related hypertension. Patients who have taken diuretics chronically before pregnancy or who are extremely sensitive to salt may derive some benefit from them (36).
TABLE 31.8 Guidelines for Treating Severe Hypertension near Term or during Labor | ||
|---|---|---|
|
TABLE 31.9 Antihypertensive Drugs Used to Treat Chronic Hypertension in Pregnant Women | ||
|---|---|---|
|
Among agents used to treat chronic hypertension in pregnancy, Aldomet (methyldopa) is safe and preferable to other drugs. Randomized trials have demonstrated that Aldomet improves fetal survival, and it is the only drug with long-term
follow-up (7.5 years), in which no difference in health, physical, or intellectual outcomes was seen (37,38). The use of β-receptor–blocking agents during pregnancy has been studied. Growth retardation, acute fetal distress, high perinatal mortality, and hypoglycemia have been found to be nearly nonexistent in controlled trials, and use of these agents are now considered nearly as safe as administration of Aldomet (38,39). Type II calcium channel blockers are potent vasodilators and have been used to treat hypertension during pregnancy. Uterine blood flow is minimally affected by nitrindipine and nifedipine, and the drug may decrease uterine contractions as a result of its smooth muscle relaxant properties; therefore, it may serve as a successful tocolytic agent (40).
follow-up (7.5 years), in which no difference in health, physical, or intellectual outcomes was seen (37,38). The use of β-receptor–blocking agents during pregnancy has been studied. Growth retardation, acute fetal distress, high perinatal mortality, and hypoglycemia have been found to be nearly nonexistent in controlled trials, and use of these agents are now considered nearly as safe as administration of Aldomet (38,39). Type II calcium channel blockers are potent vasodilators and have been used to treat hypertension during pregnancy. Uterine blood flow is minimally affected by nitrindipine and nifedipine, and the drug may decrease uterine contractions as a result of its smooth muscle relaxant properties; therefore, it may serve as a successful tocolytic agent (40).
Angiotensin-converting-enzyme (ACE) inhibitors are the agents of choice for use in nonpregnant patients who have cardiomyopathy or congestive heart failure and after myocardial infarction, but this class of antihypertensive drugs should never be used during pregnancy (41). Fetal complications include growth retardation, renal tubular dysplasia, renal failure, bone malformations (hypocalvaria, limb contractures), oligohydramnios, patent ductus arteriosis, pulmonary hypoplasia, respiratory distress, and neonatal death (42,43,44,45). The U.S. Food and Drug Administration (FDA) issued a warning against the use of ACE inhibitors during pregnancy after the first trimester (46). Most authorities favor avoiding these agents even in patients who are contemplating pregnancy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree