The Heart and Kidney Disease: Introduction
Patients with cardiovascular disease (CVD) have a high prevalence of chronic kidney disease (CKD), and information about patient kidney function should be included in clinical decision making to arrive at realistic assessments of CVD risk, to reduce the risk of acute kidney injury (AKI) associated with diagnostic and therapeutic interventions, and to adjust medications to account for altered renal pharmacokinetics. Conversely, patients with CKD are at increased risk of both common cardiovascular diseases, including hypertension, coronary artery disease, heart failure, peripheral vascular disease, arrhythmias, and sudden death, and for less common heart diseases, including pericarditis and catheter-related endocarditis.
There is extensive evidence that impaired kidney function is under-detected among the general population, hospitalized patients, high-risk patients, and patients with CVD under medical care.1-4 Cardiology patients are particularly at risk from unrecognized kidney disease because of the extraordinarily high prevalence of impaired kidney function among these patients. The reasons for the low levels of detection and awareness of CKD among clinical populations are unclear, but, as will be discussed later, increase the risk of avoidable errors in clinical decision making and management of patients with CVD.
Definition of Kidney Disease
CKD is defined by the persistence for ≥3 months of structural and/or functional abnormalities of the kidney.5 The time component of the definition accounts for nonsustained AKI; temporary physiologic abnormalities associated with medications, changes in volume status, or cardiac function; and for laboratory variation and error.
Structural abnormalities that define CKD include albuminuria, abnormal urinary sediment, and positive renal imaging tests. There is near universal agreement that urinary albumin excretion is the preferred screening test for CKD and is best measured by a calculated albumin-to-creatinine ratio (ACR) from a first voided urine specimen.6 Microalbuminuria is defined as an ACR of 30 to 300 mg/g, and anything above this level is macroalbuminuria.
The relationship between serum creatinine (Scr) and the glomerular filtration rate (GFR) is nonlinear, and substantial differences in GFR may be observed for the same Scr among individuals of differing sex, age and race. This often misleads clinicians about the presence and extent of impaired kidney function. Table 99–1 illustrates that the same Scr levels are associated with clinically important differences in levels of kidney function.7 This table underscores the importance of routine reporting by the laboratory of creatinine-based, multivariable equation estimate of GFR or, particularly for the purpose of renal drug dosing, the Cockcroft-Gault equation.8
| WF Age | 1 | 1.25 | 1.5 | BF | 1 | 1.25 | 1.5 |
|---|---|---|---|---|---|---|---|
| 45 | – | 49 | 40 | – | – | 48 | |
| 55 | – | 47 | 38 | – | 57 | 46 | |
| 65 | 59 | 46 | 37 | – | 55 | 45 | |
| 75 | 57 | 44 | 36 | – | 54 | 44 | |
| 85 | 56 | 43 | 35 | – | 52 | 43 | |
| WM | BM | ||||||
| 45 | – | – | 45 | – | – | – | |
| 55 | – | – | 52 | – | – | – | |
| 65 | – | – | 50 | – | – | – | |
| 75 | – | – | 48 | – | – | 59 | |
| 85 | – | – | 47 | – | – | 57 |
If an estimate of GFR is not provided as a routine laboratory report when ordering the Scr, free online calculators from the National Institutes of Health’s National Kidney Disease Education Program (NKDEP), or other sites, are available.9 The 4-variable Modification of Diet in Renal Disease equation:
GFR = 175 * standardized Scr1.154 * age0.203 * 1.212 (if black) * 0.742 (if female)
is the most frequently used for this purpose and has been validated in individuals aged 65 years and older, African Americans, obese individuals with a body mass index of ≥30 kg/m2, and individuals with diabetes.10 GFR estimation is imprecise above 60 mL/min/1.73 m2, and accuracy improves as kidney function declines. The Scr should be measured by a method recalibrated to be traceable to isotope dilution mass spectrometry, and the laboratory should report both a value for estimated GFR and serum creatinine.11,12
There are considerable efforts under way to improve the precision of GFR estimation through refinements of creatinine-based equations,10 substituting filtration marker like cystatin C for Scr,13 and redefining the stages of CKD.14 These efforts are exploratory and, until new evidence-based clinical practice guidelines are published, there is no reason to modify current practice.9
The imprecision of the estimated GFR at values greater than 60 mL/min/1.73 m2 is accommodated by assigning patients to broader CKD stages, which provides considerable prognostic information, as shown in Table 99–2. As CKD stage increases, hypertension, diabetes, and anemia increase in prevalence, inflammatory burden (measured by lower serum albumin and higher C-reactive protein) increases, and the risks for incident end-stage renal disease (ESRD), ischemic CVD, and all-cause mortality all increase.5,15-17 The CKD stage also provides substantial information about the risk of postoperative mortality and morbidity among patients undergoing cardiopulmonary bypass surgery and general surgery.18-20
| Description | Normal to Mild Decrease in GFR | Moderate GFR Loss | Severe GFR Loss |
|---|---|---|---|
| CKD stage | 1-2 | 3 | 4 |
| eGFR mL/min/1.73 m2 | ≥60 | 30-59 | 15-29 |
| Cardiovascular risk factors | |||
| Hypertension5 | 40% | 55% | 77% |
| Diabetes16 | 3.1%-6.5% | 16.8% | 22.8% |
| C-reactive protein >0.21 mg/dL16 | 25%-30% | 48.7% | 57.7% |
| Hemoglobin <13 g/dL15 | 4% | 7% | 29% |
| Outcomes | |||
| Five-year ESRD rate17 | 1.1% | 1.3% | 19.9% |
| Five-year mortality rate17 | 19.5% | 24.3% | 45.7% |
| Three-year CVD event rate21 | 2.1% | 4.8% | 11.4% |
| Acute coronary syndrome18 | |||
| Prevalence among patients | 59.3% | 31.9% | 8.8% |
| Risk of 7-month mortality AMI | 1 | 2.1 (1.04, 4.3)a | 4.6 (2.1, 9.9)a |
| Risk of 7-month mortality UA | 1 | 8.8 (1.2, 67.2)a | 24.6 (3.0, 202.4)a |
| Acute myocardial infarction19 | |||
| Prevalence among patients | 28.5% | 43.5% | 30% |
| Risk of 1-year mortality AMI | 2.3% | 9.4% | 24.2% |
| Coronary artery bypass surgery20 | |||
| Prevalence among patients | 73.9% | 24.1% | 2.0% |
| Risk of 30-day postoperative mortality | 1.3%-1.8% | 4.3% | 9.3% |
| Postoperative stroke | 0.9%-1.3% | 2.4% | 3.5% |
| Prolonged ventilation | 5.3%-6.1% | 11.1% | 19.7% |
| Deep sternal infection | 0.4% | 0.6% | 0.9% |
Association between Chronic Kidney Disease and Cardiovascular Disease
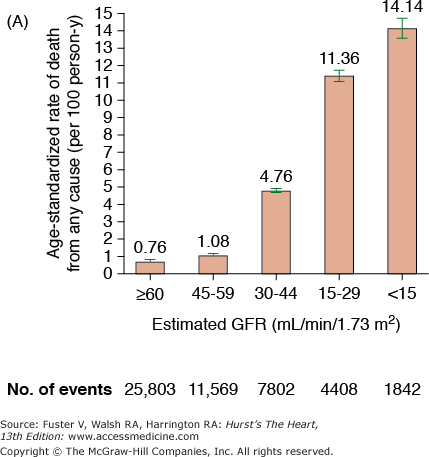
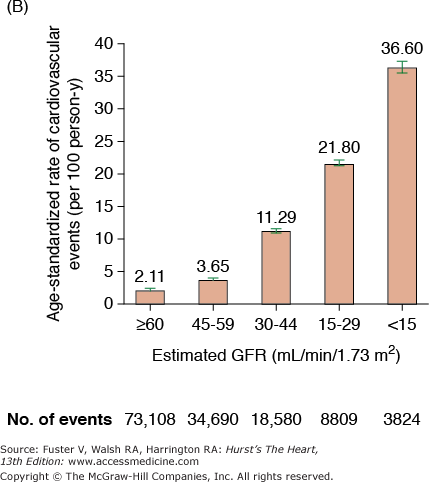
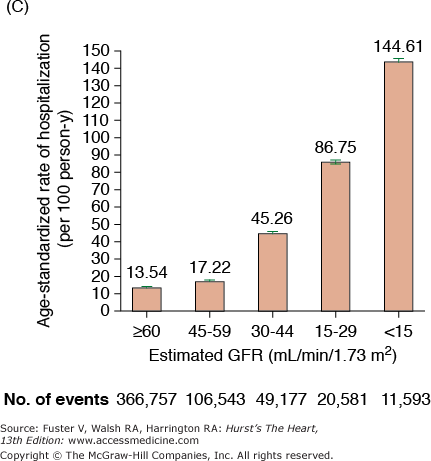
CKD is associated with increased risk of heart disease (see Table 99–2), and CVD is the most frequent cause of death in patients with ESRD.20 The cardiovascular mortality rate in ESRD patients on dialysis is approximately 10 to 30 times higher than in the general population adjusted for age, race, and sex. Similar marked and graded increases in risk of all-cause death, death due to CVD, and risk of hospitalization with declining GFR have also been reported for the general population (Fig. 99–1).20,21 Among patients with less severe stages of CKD, the risk of all-cause and CVD-specific mortality,22 arrhythmia-related deaths, and sudden death is substantially increased.23,24 The association between CVD, CKD, and adverse outcomes is quite strong, and a recent review found that positive associations between all stages of CKD and increased risk of CVD were observed in all but 2 of 49 populations.25
Patients with CKD are at increased risk of developing CVD.26 A recent analysis of the Cardiovascular Health Study found that the 3-year predicted probability of incident CVD increased in a monotonic fashion as GFR declined from values above 90 mL/min/1.73 m2 to 30 mL/min/1.73 m2, and this increased risk persisted after controlling for other risk factors.27 The unadjusted risks for incident CVD were 15% and 40% among individuals with GFR ≥90 and 30 mL/min/1.73 m2, respectively. Adjustment for CVD risk factors reduced the respective risks to 15% and 22%. Analyses of the Cardiovascular Health Study,28 the Health ABC study,29 managed care populations,17 and a follow-up study of the ALLHAT population30 have reported similar associations. Although the increased risk of incident CVD among patients with CKD is independent of established and novel CVD risk factors, the prevalence of these risk factors substantially increases as kidney function declines.31
The prevalence of CKD in the general population based on persistent microalbuminuria or estimated GFR less than 60 mL/min/1.73 m2 is estimated to be 13.1%, and because of the rapid aging of the US population, this rate has increased 30% between 1988 and 2004.32 The reported prevalence of CKD among clinical populations is highly variable and can vary from less than 10% to more than 60% of a population of CVD patients, with an average prevalence of 30%.33 The presence of CVD also increases the risk of developing ESRD.34 For example, hypertensive male veterans are at a two-fold increased risk of developing ESRD after a new myocardial infarction and five-fold increased risk after incident heart failure.35
Acute Kidney Injury
Patients hospitalized for CVD34 and related diagnostic,36 interventional,37 and surgical procedures38 are at increased risk of AKI, whether they have normal kidney function or CKD. The definition of AKI has been formalized by the Acute Dialysis Quality Initiative consensus-based classification known as RIFLE (risk, injury, and heart failure; loss; and end stage kidney disease).39 The incidence of AKI has increased among hospitalized patients from 15 to 35 cases per 1000 discharges between 1992 and 2001,40 and recent studies have shown that the stages or levels of AKI defined by the RIFLE criteria are predictive of increased risk of mortality and dialysis-dependent kidney failure in critically ill patients and those undergoing cardiac surgery.41,42
It is important to emphasize that this increased risk of adverse outcomes of AKI is observed even with small increments of serum creatinine on the order of 0.5 mg/dL among individuals with and without CKD before admission. Risk factors for AKI among patients with CVD include presence of CKD, older age, diabetes mellitus, proteinuria, heart failure, exposure to radiocontrast dye, surgical and percutaneous revascularization, and admission to acute care units.43 At present there are no risk stratification equations that predict risk of AKI that have been extensively validated, and thus clinicians must maintain a low threshold for the identification of at-risk patients. AKI is also a common complication after cardiac surgery, and even minor elevations in Scr after cardiac surgery increases mortality risk.44 The need for acute dialysis after cardiac surgery is not only associated with increased hospital mortality and morbidity, but also with a significant reduction in long-term survival in discharged patients.45 Although a variety of pharmacologic agents such as low-dose dopamine,46 fenoldopam,47 N-acetylcysteine,48 and, more recently, nesiritide49 have been reported to prevent AKI after cardiac surgery, none of these agents has proven benefit in reducing need for acute dialysis from randomized prospective clinical trials.
Contrast-induced nephropathy (CIN) represents a potentially preventable cause of AKI among CVD patients. CIN is the third most common cause of hospital-acquired AKI.43 The risk of CIN is a particular concern for patients with CVD undergoing diagnostic and therapeutic angiography.36 Typically, Scr begins to rise within 24 to 72 hours after a radiocontrast study, and renal dysfunction is typically brief (approximately 5-7 days), unless there is preexisting renal damage. Fewer than 1% of those who develop CIN with baseline GFR of >60 mL/min/1.73 m2 require dialysis, but in patients with GFR of ≥30 mL/min/1.73 m2, the need for dialysis approaches 2% to 8%.50
To prevent the development of CIN, patients at risk for this complication need to be identified before the planned contrast administration.Table 99–3 details common risk factors for CIN. At present there are multiple proposed risk stratification models to predict the occurrence of CIN, but none has been widely accepted, and it is prudent to consider any patient with either CVD or CKD as being at high risk for CIN. For high-risk patients, the Consensus Panel for CIN recently published recommendations for prevention and management of CIN51 that call for preprocedure recognition of risk and maintenance of adequate hydration before, during, and after the procedure. A standardized protocol of hydration with 0.45% saline 12 hours before and 6 hours after contrast exposure has been proven effective in reducing the risk of CIN and should be used routinely. Recent comparison and meta-analyses of sodium bicarbonate and N-acetylcysteine have failed to provide strong and consistent evidence of a benefit of these agents.52-54 In high-risk patients, consideration should be given to obtaining a serum creatinine between 24 hours and more than 72 hours after contrast exposure. There are not good controlled studies available to justify the practice of immediate hemodialysis after contrast media administration for ESRD patients. However, in certain clinical situations, including pulmonary congestion or hyperkalemia after contrast administration, immediate dialysis is indicated.
| Risk Factor | Odds Ratio (95% Cl) |
|---|---|
| Patient-related | |
| Preexisting renal dysfunction | |
| Serum creatinine level: | |
| 1.2-1.9 mg/dL (106-176 mmol/L) | 2.42 (1.54-3.79) |
| 2.0-2.9 mg/dL (177-264 mmol/L) | 7.37 (4.78-11.39) |
| ≥3.0 mg/dl (265 mmol/L) | 12.82 (8.01-20.54) |
| Diabetes mellitus | 5.47 (1.40-21.32) |
| Age (1-year increment) | 1.02 (1.01-1.03) |
| Congestive heart failure | 1.53 (1.21-2.10) |
| Hypertension | 1.20 (1.06-1.36) |
| Low effective circulatory volume | 1.19 (0.72-1.95) |
| Myocardial infarction | 1.85 (1.31-2.63) |
| Use of intra-aortic balloon pump | 1.94 (1.08-3.49) |
| Other | |
| Osmolality and content of contrast medium in patients with preexisting renal dysfunction (low vs high osmolality) | 0.50 to (0.36-0.68) |
| Volume of contrast medium (per 100 mL) | 1.12 (1.02-1.23) |
Management of Cardiovascular Risk Factors and CKD
CKD and CVD share common risk factors. The higher prevalence of CVD in patients with renal disease may be the consequence of the high prevalence of hypertension, diabetes, aging, and dyslipidemias. There is increasing concern that interventions to reduce the risk of incident and recurrent CVD, including agents blocking the renin-angiotensin-aldosterone system (RAAS), β-blockers, aspirin, platelet inhibitors, thrombolytics, or percutaneous intervention, may be underutilized among patients with CKD, despite their utility in this population.55,56
The kidney is central to regulation of arterial pressure. Increased prevalence of hypertension begins in CKD stage 3, and almost 80% of CKD patients have systemic hypertension before beginning dialysis therapy.57 After the onset of dialysis therapy, hypertension is almost universal among ESRD patients. The pathogenesis of hypertension includes both an expanded extracellular fluid volume (ECV) and increased peripheral vascular resistance mediated by increased activity of the sympathetic nervous system and the renin-angiotensin axis.58-60
Drug therapy for hypertension in CKD stages 1 to 4 is based on strong evidence that inhibiting RAAS axis is effective even in advanced CKD, is cardioprotective, reduces risk of CKD progression in both diabetic and nondiabetic CKD, and is effective in African Americans.61-64 This evidence has been incorporated into recommendations of the American Diabetes Association (ADA)65 and the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VII).66
Both the ADA and JNC VII call attention to the rises in Scr and/or potassium that are a physiologic consequence of the use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs). A meta-analysis of 12 randomized clinical trials found that participants with CKD often experienced an increase in Scr of up to 30% that was not associated with progressive loss of kidney function, and it did not attenuate the renal protection of these agents.61 Hyperkalemia can be detected by assessment of serum electrolytes before and 1 to 2 weeks after the initiation of therapy67 and may be managed by reducing RAAS blocker dose, restricting dietary potassium, avoiding prescription potassium supplement, minimizing the use of nonsteroidal anti-inflammatory agents, and minimizing the use of potassium-sparing diuretics. Similarly, it is recommended that Scr be checked within a similar time frame. An elevation over baseline Scr of greater than 25% should warrant further evaluation to ensure that volume status is adequate, blood pressure has not been excessively lowered, and that other agents that might concurrently lower GFR, such as nonsteroidal anti-inflammatory agents, have not been introduced into the therapeutic program. In the absence of readily reversible causes for an excessive rise in creatinine, one can stop the ACEI/ARB with full expectation that kidney function will return to baseline.68,69 It is to be emphasized that rarely is it necessary to stop using RAAS blocking therapy in patients even with the most advanced CKD.
Both the ADA and JNC VII recommend a therapeutic target of 130/80 mm Hg. Achieving this degree of blood pressure reduction in individuals with CKD typically requires the use of two to three antihypertensive agents, including a RAAS blocker and a diuretic.68 It should be noted that thiazide diuretics retain their efficacy in CKD stage 3.70
A cornerstone of correcting hypertension in patients with CKD is the reduction in dietary sodium intake. The JNC VII recommends the adoption of the Dietary Approaches to Stop Hypertension (DASH) diet, which is a diet rich in fruits, vegetables, and low-fat dairy products with a reduced content of dietary cholesterol as well as saturated and total fat.66 This diet has a higher potassium composition that the regular American diet, and this must be taken into account when initiating or changing diet on the background of RAAS blocker use. A target of no more than 100 mmol of dietary sodium (2.4 g of sodium) per day should be prescribed and, if possible, the patient should be referred to a dietician experienced in working with patients with CKD.
In patients who have persistent hypertension, despite adequate blockade of the RAAS, calcium channel blockers and β-blockers are logical additions. If these are ineffective, clonidine or minoxidil can be used. The dosage of antihypertensive drugs (like other medicines) must be adjusted for the degree of renal failure.
One of the important CVD risk factors in uremic patients is the presence of dyslipidemia.71 Serum lipid abnormalities are more common in patients with CKD than in the general population, but the pattern differs.72 All patients with CKD manifest a secondary form of dyslipidemia that mimics the atherogenic dyslipidemia of insulin-resistant patients. This is characterized by an increase in serum triglyceride (TG), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL), with unchanged total cholesterol (TC) and low high-density lipoprotein (HDL). However, in patients with nephrotic syndrome, LDL and TC are markedly elevated. Hemodialysis (HD) patients usually have “normal” TC and LDL levels, whereas lower HDL and higher serum TG levels are observed. Patients treated with chronic peritoneal dialysis (CPD) have similar pattern of dyslipidemias except they are more likely to have elevated LDL levels. Current evidence-based recommendations from the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults call for lowering elevated LDL concentration with a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) in patients with dyslipidemia.73 A recent meta-analysis of 26 studies with more than 25,000 participants that enrolled adult patients with CKD stages 3 to 4 (GFR <60 mL/dL/min) found that statin therapy, when compared with placebo, significantly reduced levels of total cholesterol by 41.48 mg/dL (95% CI, −49.97 to −33.99), LDL cholesterol by 42.38 mg/dL (95% CI, −50.71 to −34.05), TG by 28.71 mg/dL (95% CI, −48.55 to −8.87), and all-cause mortality (relative risk [RR] 0.81; 95% CI, 0.74 to 0.89) and cardiovascular mortality (RR 0.80; 95% CI, 0.70 to 0.90).74 Statin therapy was associated with a reduction in 24-hour urinary protein excretion of 0.73 g/24 h (95% CI, −0.95 to −0.52). In a small number of these studies (n = 11; 548 patients) in which kidney function was assessed, a nonsignificant increase in creatinine clearance of 1.48 mL/min (95% CI, −2.32 to 5.28) was noted.74 Adverse event rates in this meta-analysis for rhabdomyolysis, elevated liver enzymes, and withdrawal rates were comparable among patients receiving either statins or placebo.
A separate meta-analysis by the same Cochrane group found that statin therapy use was also effective in lowering lipid levels and had an acceptable safety profile in stage 5 CKD patients on hemodialysis.75 Two recent large-scale randomized clinical trials addressing this issue in stage 5 CKD patients, the Deutsche Diabetes-Dialyse-Studie (4D) Study Group trial76 and the AURORA Study Group trial,77 both failed to observe reductions in cardiovascular event rates among statin-treated patients, despite a significant reduction in LDL levels in this group.
Thus current evidence supports the use of statin therapy in stages 1 to 4 CKD patients with dyslipidemia to reduce the risk of all-cause and cardiovascular mortality and as an adjunct to reduction of proteinuria. Their role in the treatment of stage 5 CKD remains uncertain. The NCEP III guidelines and the National Kidney Foundation clinical practice guidelines for managing dyslipidemias provide recommendations for classifying and treating lipid abnormalities in patients with CKD.78
Calcium phosphate deposition, in the form of bioapatite, can occur in blood vessels, myocardium, and cardiac valves in two distinct patterns, intimal calcification of atherosclerotic lesions, and medial calcification, or Mönckeberg sclerosis.79 The increased incidence and severity of vascular calcification in uremia is attributed to the abnormalities of mineral metabolism, which are common in CKD patients, especially those on dialysis. Meta-analyses have clearly shown that the presence and severity of arterial calcification is associated with adverse clinical outcomes, including myocardial infarction, congestive heart failure, endocarditis, valvular heart disease, and death among general patient populations and those with advanced CKD.80,81 A meta-analysis of 30 studies with more than 200,000 subjects reported that prevalent arterial calcification was associated with a nearly five-fold increased risk for all-cause mortality, four-fold risk for cardiovascular mortality and coronary events, two-fold risk for stroke, and 3.41 (CI, 2.71-4.30) times increase for any cardiovascular event. Subgroup analyses of patients with stage 5 CKD (ESRD) were associated with a more than six-fold increased risk of CVD events.82
Increased vascular calcification is thought to be a consequence of dysregulation of calcium and phosphate homeostasis in patients with CKD that depends on two major regulatory hormone systems, parathyroid hormone (PTH) and active 1,25-dihydroxyvitamin D (calcitriol). As GFR falls, there is reduction in the amount of filtered and excreted phosphate, resulting in elevation of serum phosphate concentration. Any increase in serum phosphorus levels reduces ionized calcium, which in turn stimulates secretion of PTH (secondary hyperparathyroidism), resulting in improved phosphorus excretion.
Observational studies have shown a strong association between high serum phosphorus and serum calcium-phosphorus product,83,84 high serum PTH levels,85 and low vitamin D levels86,87 with all-cause and cardiac mortality among individuals with CKD. Adequate control of serum phosphorus remains a cornerstone in the clinical management of patients with CKD and ESRD, not only to attenuate the progression of secondary hyperparathyroidism, but also possibly to reduce the risk for vascular calcification. However, phosphate binders are often necessary to limit dietary absorption of phosphorus. For patients with high serum calcium-phosphorus product, a non–calcium-containing phosphate binder, such as sevelamer hydrochloride or lanthanum carbonate, should be selected to reduce the amount of ingested calcium. However, these agents are significantly more expensive than calcium salts, which may contribute to patient noncompliance. In contrast, if the serum calcium is low, calcium carbonate or calcium acetate are cheaper and effective binders. Calcimimetics are agents that bind the calcium-sensing receptor of the parathyroid gland, resulting in diminished PTH secretion without increasing serum calcium and phosphorus levels.88 Although randomized clinical trials consistently show benefit of phosphate binders, vitamin D, and cinacalcet, a recently released calcimimetic, on surrogate outcomes like calcium, phosphate, PTH abnormalities, and vascular calcification, there is no convincing evidence that these interventions reduce hospitalization, fracture, cardiovascular events, or death.89
Anemia begins early in the course of CKD and is almost uniform among CKD stage 5 patients treated with dialysis.90 An association between lower hemoglobin and risk of CVD among stage ≥3 CKD patients is well characterized.91 Anemia is also a risk factor for stroke and heart failure, and these associations persist after controlling for other risk factors.92,93 Although there is no evidence from clinical trials to support a reduction in mortality from correction of anemia in CKD stages 3 and 4 patients,94,95 treatment to a target hemoglobin concentration of between 10 and 12 g/dL is associated with improvement in physical activity, vitality, and fatigue96,97; reduction in risk of left ventricular hypertrophy98; and improved outcomes in heart failure.99
Approximately, one-third of individuals with types 1 and 2 diabetes will develop diabetic kidney disease, and approximately 45% of all CKD patients who began maintenance dialysis had diabetes. As in the general population, diabetic dialysis patients have more cardiovascular morbidity and mortality and all-cause mortality compared with dialysis patients without diabetes. There is strong evidence that tight glycemic control in types 1 and 2 diabetes mellitus, reduction of blood pressure to below 130/80 mm Hg with either ACEIs or ARBs, and dietary protein restriction to the recommended daily allowance of 0.8 g/kg/day reduce the risk of progressive kidney disease and risk of CVD. These therapeutic principles should be considered when managing cardiovascular risk in patients with types 1 and 2 diabetes mellitus.100
There is growing interest in the role of albuminuria, obesity, elevated uric acid, insulin resistance, and the metabolic syndrome as mediators of progressive kidney disease, similar to the role of these as risk factors for CVD.101-107 Further, patients with CKD have an increased prevalence of obesity, which is also associated with increased risk of ESRD.108 There is some evidence that weight reduction decreases the hyperfiltration and renal blood flow associated with obesity and that it may ameliorate risk of progressive kidney disease.109 Weight reduction should be a standard component of the dietary prescription of obese patients with CKD and CVD. However, caution should be exercised in caloric restriction among ESRD patients on hemodialysis where obesity has been associated with decreased mortality after the onset of renal replacement therapy.110
Clinically significant hypotension occurs in approximately 10% to 30% of HD treatments.111 Intradialytic hypotension (IDH) is often defined as a systolic blood pressure <100 mm Hg or a blood pressure drop of >20 mm Hg during a dialysis session with concomitant symptoms of dizziness, blurred vision, cramps, and fatigue.112 Usually the consequences are minor, but cerebrovascular insufficiency and/or cardiovascular instability (myocardial ischemia and arrhythmias) can occur.113,114 These complications of hypotension may account at least in part for the “U-shaped” relation between systolic blood pressure and cardiovascular mortality in HD patients. The relative death rate for patients with postdialysis systolic blood pressures less than 110 mm Hg was double the rate for those with a postdialysis systolic blood pressure of 140 to 149 mm Hg.115
The main cause of intradialytic hypotension is hypovolemia due to an imbalance between the amount of fluid removed and the refilling capacity of the intravascular compartment. Several methods have been employed to reduce the incidence of hypotension during dialysis. Those include withholding antihypertensive medications on dialysis days, restricting interdialysis weight gain, avoiding eating on dialysis, modifying the dialysate sodium concentration during the dialysis treatment, and cooling of dialysate.115 Other strategies for preventing hypotension include removing ECV at a slower rate and using online hematocrit monitoring to detect a sudden decrease in blood volume.116 For patients unresponsive to above measures, a trial of midodrine 2.5 to 10 mg given 15 to 30 minutes before dialysis is a consideration.112