The Heart and Infectious Diseases
Andrew Boyle
Overview
Infectious myocarditis is an important etiology of both fulminant and chronic heart failure. Systemic infections such as the human immunodeficiency virus (HIV) and Chagas disease are common causes of profound heart failure, particularly in developing countries. A method for accurately diagnosing infectious myocarditis premortem is elusive, and diagnosis largely relies on circumstantial evidence such as rising serum antibody titers. Newer techniques such as gene amplification by reverse transcription polymerase chain reaction (rt-PCR) and nucleic acid hybridization of right ventricular endomyocardial biopsy (EMB) tissue suffer from a lack of sensitivity. Furthermore, once a diagnosis is made, treatment is mainly focused on hemodynamic support and symptom relief as opposed to counteracting the pathologic organism. Nevertheless, full recovery of cardiac function is common, although not uniform.
Clinical Presentation in Infectious Myocarditis
There is a wide spectrum of clinical presentations of infectious myocarditis, ranging from a clinically silent syndrome with complete recovery to acutely or chronically decompensated irreversible heart failure leading to either death or cardiac transplantation. The symptoms of mild to moderate infectious myocarditis are relatively nonspecific, consisting of fevers, sweats, or chills associated with mild dyspnea and palpitations. In severe cases of infectious myocarditis, symptoms relate to hypoperfusion and cardiac congestion. Chest pain is relatively infrequent and usually results from accompanying pericarditis. The signs of infectious myocarditis are also nonspecific and include frequent atrial and ventricular ectopy, sinus tachycardia, and, in severe cases, ventricular arrhythmias and volume overload. Varying degrees of atrioventricular heart block may develop transiently and usually resolve spontaneously and completely (1). In the majority of cases, the initial infection remains completely unrecognized and full recovery of cardiac function ensues. However, for unclear reasons, in a minority of patients, the infectious insult leads to progressive myocardial dysfunction either acutely or, more commonly, many years later. This is believed to be one of the more common etiologies for dilated cardiomyopathy (DCM), accounting for 12.8% of all cases (2). Most commonly (in 51.2% of all cases), no etiology is discovered, and the cardiomyopathy is thus termed idiopathic.
Diagnosis of Infectious Myocarditis
In light of the lack of specific signs and symptoms for infectious myocarditis, an accurate and early diagnosis of this condition relies on a high clinical suspicion by the practitioner. Often the earliest evidence of active myocarditis is nonspecific changes on the resting electrocardiogram. These changes usually involve the ST segment and the T wave, but in severe cases, significant intraventricular conduction delays can develop either transiently or permanently. A pseudoinfarction electrocardiographic pattern with Q waves and ST-segment elevation offers a particularly poor prognosis, often with a rapidly fatal course (3). Abnormal QRS morphology and left-bundle-branch block are also indicative of a worse prognosis, although usually from progressive congestive heart failure over a more protracted time course (1). There electrocardiographic changes are obviously more helpful when a baseline electrocardiogram is available for comparison.
Serum troponin levels (4) can be elevated as a reflection of ongoing myocardial necrosis from the inflammatory process. The degree of their elevation, indeed whether they are elevated at all, is a function of both the severity of the inflammatory insult as well as its acuity. Both the sensitivity and specificity of these markers of myonecrosis are particularly poor.
A documented fourfold rise in serum viral antibody titers from the acute to the convalescent stage of the infection is indirect evidence of viral infection, which does not assist in the management of the patient in the acute phase of the illness
(5). A significant rise in viral-specific immunoglobulin M (IgM) antibody titers is an earlier serologic finding, although it is still not rapid enough to have therapeutic implications.
(5). A significant rise in viral-specific immunoglobulin M (IgM) antibody titers is an earlier serologic finding, although it is still not rapid enough to have therapeutic implications.
Echocardiography is of limited use in the diagnosis of acute infectious myocarditis and serves mainly to confirm left ventricular systolic dysfunction as well as to eliminate other potential etiologies, particularly valvular cardiomyopathies and anatomic abnormalities such as atrial and ventricular septal defects. Gadolinium-enhanced magnetic resonance imaging (MRI) has proven to be relatively sensitive in the detection of acute myocarditis (6). However, it lacks specificity in that abnormal myocardial enhancement reflects only active inflammation regardless of its etiology, including after a myocardial infarction (MI) and various other cardiomyopathic processes.
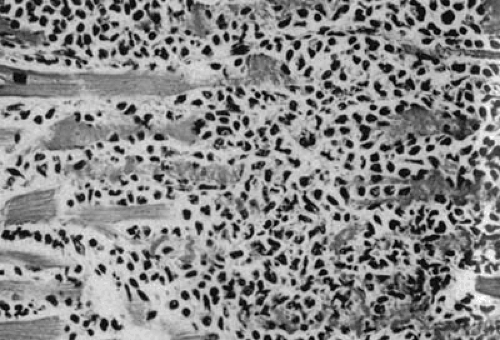
The gold standard for diagnosis of acute viral myocarditis remains the EMB, despite its many imperfections. The Dallas criteria were created by a panel of cardiac pathologists in 1986 to standardize the pathologic requirements for a diagnosis of IM (7). In brief, a diagnosis of IM in this classification system requires the simultaneous finding of lymphocyte infiltration and myocyte necrosis (Fig. 35F.1). EMB samples in which one criterion is met but not the other are deemed borderline myocarditis. Despite this attempt to create uniformity in histologic diagnosis, there remains significant interobserver variability (8). Furthermore, lymphocytic myocarditis is a patchy disease and is unevenly distributed throughout the myocardium (9). In some patients, the pathologic myocardium is not easily accessible by the bioptome, which, by definition, is limited to sampling subendocardial tissue. Even when the inflammatory process involves the subendocardium, there is a tremendous sampling error when only three to five biopsies are obtained (10). The rate of false-negative EMB is as high as 83% for individual biopsies and 55% when five biopsy samples are obtained in a single patient with autopsy-proven IM (9). In addition, there is a high rate of false-positive EMB because of the presence of small numbers of lymphocytes normally present in the interstitium and because of the difficulty in distinguishing among lymphocytes, interstitial macrophages, fibroblasts, endothelial cells, and pericytes by light microscopy (11). Newer techniques that have been developed to detect myocardial viral genome products in EMB samples using cloned DNA fragments complementary to the viral genome (in situ DNA hybridization) and rt-PCR have generally been successful (12,13). However, these techniques rely on having adequate biopsy samples and thus suffer from significant sampling errors as previously discussed. Furthermore, they have yet to be implemented in the clinical setting because there are few virus-specific therapies that could be initiated should a specific etiology be identified.
Because of the relatively low sensitivity and specificity of all the aforementioned diagnostic studies, the diagnosis of IM at this time and for the foreseeable future remains mainly a clinical one.
Etiologic Agents of Infectious Myocarditis
Viruses
Enteroviruses
The enteroviruses are a group of viruses pathogenic to the gastrointestinal and upper respiratory tracts (Table 35F.1). Coxsackie B virus (CBV) appears to be the most common pathogen. Serologic studies have revealed that 36% of all adults with acute myocarditis have demonstrable rises in either their serum anti-CBV antibody titers or IgM (14,15). The full epidemiologic impact of CBV-induced myocarditis is difficult to assess in light of the ubiquitous nature of this organism as evidenced by the high rate of similar findings in control populations (15).
Elements of the CBV genome have also been detected using rt-PCR in EMB of patients who met the Dallas criteria for diagnosis of acute myocarditis (16). The specificity of this finding is questionable, however, due to the unexpectedly high finding of enteroviral RNA in autopsy cases without cardiac disease (16). Furthermore, delineation of the exact viral species using this method is difficult due to significant nucleic acid sequence homology at the 5′-untranslated region of the RNA of all enteroviruses (17). However, probes do exist to species-specific regions of the RNA, which are thus able to differentiate among various species using in situ hybridization.
Elements of the CBV genome have also been detected using rt-PCR in EMB of patients who met the Dallas criteria for diagnosis of acute myocarditis (16). The specificity of this finding is questionable, however, due to the unexpectedly high finding of enteroviral RNA in autopsy cases without cardiac disease (16). Furthermore, delineation of the exact viral species using this method is difficult due to significant nucleic acid sequence homology at the 5′-untranslated region of the RNA of all enteroviruses (17). However, probes do exist to species-specific regions of the RNA, which are thus able to differentiate among various species using in situ hybridization.
TABLE 35F.1 The Pathogens in Acute Infectious Myocarditis | ||
|---|---|---|
|
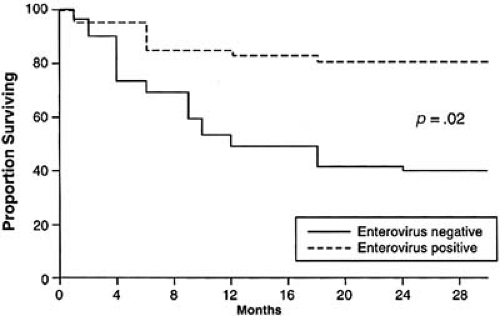
In the vast majority of patients infected with CBV, cardiac manifestations are not appreciated, although cardiac involvement has been estimated to be as high as 5.3% (18). In most cases, the signs and symptoms of infection are benign and resolve spontaneously. Some investigators have nevertheless proposed that some cases of nonischemic DCM may represent the end stage of either a previously detected (19) or undetected (20) enteroviral infection. Much of this speculation has arisen as a result of the frequent, although not uniform, detection of enteroviral RNA in EMB from patients with nonischemic DCM without clinical evidence of IM (16). In addition, patients with DCM in whom persistent enteroviral RNA can be detected in their EMB have a much worse clinical prognosis (Fig. 35F.2). However, due to the high prevalence of enteroviral infections in the community, these studies provide only circumstantial evidence of a possible link between enteroviral myocarditis and DCM without offering definitive proof. In fact, in one study, enteroviral RNA was detected more frequently in control patients undergoing either heart or lung transplantation for ischemic heart disease or primary lung disease than in patients with DCM (20). However, compelling evidence of a protease encoded by CBV mediating degradation of cardiac dystrophin in mice, thereby reducing myocardial structural integrity, suggests a potential pathogenic role for enteroviruses in DCM in humans (21,22).
Adenovirus
The adenovirus genome has recently been demonstrated in EMB and autopsy samples of myocardium from children and adults with acute myocarditis (23). It was found in a much greater proportion of patients than previously expected, although it may be less pathogenic than enteroviruses (24). The discovery of a common myocardial receptor, the so-called common CBV-adenovirus receptor, suggests a common portal of entry for these viruses and may explain their prominence as causative agents in acute myocarditis and DCM (25). The fact that this receptor appears to be upregulated in patients with DCM compared to controls is also supportive of this theory, albeit not conclusive (26).
Herpes Viruses
Cytomegalovirus (CMV) is readily identified in infected cells due to its characteristic microscopic nuclear and paranuclear inclusion bodies. CMV is a ubiquitous organism that, in the immunocompetent host, commonly produces an asymptomatic primary infection, as evidenced by the presence of circulating anti-CMV antibodies in the majority of the population (27). CMV may remain latent intracellularly indefinitely in the host waiting to become reactivated and lead to a systemic infection if the patient becomes immunocompromised, usually as a result of HIV infection, neoplasia, immunosuppression for rheumatologic or pulmonary disease, or posttransplantation. One component of this generalized infection may include CMV myocarditis, which can be adequately treated with ganciclovir (28). After cardiac transplantation, CMV has also been associated with accelerated cardiac allograft vasculopathy, which is generally believed to be reflective of chronic allograft rejection (29). This hypothesis also remains controversial because others have been unable to confirm this finding (30). Even more intriguingly, however, treatment of cardiac transplant recipients immediately postoperatively with ganciclovir appears to lower the incidence of allograft vasculopathy, regardless of whether or not they developed a systemic CMV illness (31).
Other Herpes Viruses
Epstein-Barr virus (EBV) can rarely cause symptomatic myocarditis (32). EBV has also been implicated in the development of posttransplantation lymphoproliferative disease (PTLD) after cardiac transplantation (33), which is a life-threatening complication often treated by lowering the patient’s immunosuppression and possibly systemic chemotherapy.
Human Immunodeficiency Virus
Acquired immunodeficiency syndrome (AIDS) is the end-stage of HIV infection. As the number of worldwide HIV infections has reached epidemic proportions and because advances in medical therapies have enabled prolonged patient survival from both AIDS and various opportunistic infections, the cardiac manifestations of this disease have become more apparent. These cardiac manifestations include direct effects of HIV itself and effects resulting from opportunistic infections that arise as a consequence of an immunosuppressed state.
EMB-proven acute or chronic lymphocytic myocarditis is a frequent finding in patients with AIDS (35). Most frequently, this is a consequence of a superimposed opportunistic infection. HIV RNA and DNA has been detected in such biopsies but is usually very sparse, most often in areas of myocardium without an inflammatory infiltrate or ongoing myonecrosis, and is frequently associated with other opportunistic pathogens (36). A causal relationship has therefore not been firmly established between HIV and myocarditis. It must be noted, however, that no pathogen is identified in a large percentage of cases of AIDS-associated cardiomyopathy, a finding not dissimilar from that of patients whose cardiomyopathy is unrelated to AIDS. Some investigators have proposed that AIDS-associated cardiomyopathy may result from antiretroviral therapy (37). Regardless
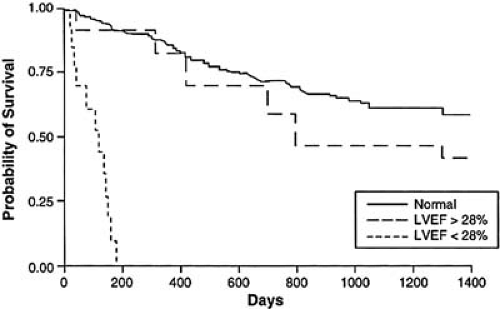
of the etiology of the cardiomyopathic process associated with AIDS, the diagnosis is a harbinger of a poor clinical prognosis, irrespective of the adequacy of the anti-HIV therapy itself (Fig. 35F.3).
of the etiology of the cardiomyopathic process associated with AIDS, the diagnosis is a harbinger of a poor clinical prognosis, irrespective of the adequacy of the anti-HIV therapy itself (Fig. 35F.3).
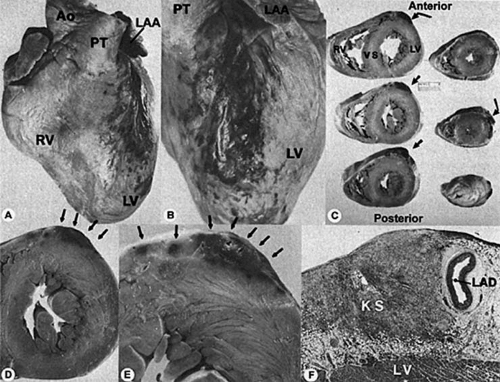
Cardiac neoplasms also develop in patients with HIV. Cardiac Kaposi sarcomas are not usually isolated to the heart, but rather are part of a widely metastatic process (38). They are usually asymptomatic, and most cases are discovered unexpectedly at autopsy (Fig. 35F.4). Cardiac non-Hodgkin B-cell lymphoma is also usually associated with a widely disseminated malignant process in HIV patients, but can be a primary lesion as well (39).
Accelerated coronary atherosclerosis has also been observed at autopsy in relatively young HIV patients, although a causal relationship has yet to be established (40). Protease inhibitors, antiretroviral agents used to treat patients with HIV, have been implicated in the development of coronary atherosclerosis (41), likely as result of profound deleterious effects on lipid profiles and insulin metabolism. A recent short-term follow-up of Veteran’s Administration patients with HIV failed to demonstrate an increase in cardiovascular (CV) events in patients treated with protease inhibitors (42). Given the potentially significant lag time between the development of hyperlipidemia and insulin resistance and the development of clinically significant cardiovascular disease, it would seem prudent to carefully manage all CV risk factors in all patients exposed to protease inhibitors. Nevertheless, protease inhibitors should not be withheld from HIV patients due to their potential cardiovascular complications given the dramatic improvement in survival and reduced HIV viremia with these agents. Statin therapy is appropriate for hyperlipidemic HIV patients, although lovastatin
and simvastatin are not recommended due to their interactions with protease inhibitors at the cytochrome P450 enzyme system (43). Care must also be taken when an HIV patient is receiving antifungals or macrolide antibiotics for either prophylaxis or treatment against a variety of opportunistic infections because these may also interact with the cytochrome P450 system.
and simvastatin are not recommended due to their interactions with protease inhibitors at the cytochrome P450 enzyme system (43). Care must also be taken when an HIV patient is receiving antifungals or macrolide antibiotics for either prophylaxis or treatment against a variety of opportunistic infections because these may also interact with the cytochrome P450 system.
Other Viruses
Several other viruses have also been proven to rarely cause acute myocarditis including hepatitis B virus (44), hepatitis C virus (45), influenza A (46) and B (47) viruses, rabies virus (48), and parvovirus B19 (49). Cases of mumps (50), measles (51), and rubella (52) myocarditis have all been reported, and fetal rubella infection, as a result of early gestational maternal transmission, can lead to multiple congenital heart defects. However, mumps, measles, and rubella have largely been eradicated as a result of widespread childhood immunization programs.
Bacteria
Rheumatic Fever
Acute rheumatic fever is characterized by a systemic inflammatory response approximately 3 weeks after an untreated group A streptococcal pharyngeal infection but never after a cutaneous infection. The incidence of acute rheumatic fever in the developed world is on the decline in light of widespread antistreptococcal antibiotic therapy for upper respiratory tract infections and general improvements in living conditions. The same is not true in the developing world. Appropriate antibiotic therapy during the preceding pharyngeal infection essentially eliminates the future risk of developing rheumatic fever (53).
The diagnosis of acute rheumatic fever is mainly a clinical one using the revised Jones criteria of 1992 (Table 35F.2). Evidence of two major criteria or one major criterion and at least two minor criteria are required for a presumptive diagnosis of rheumatic fever, which is enhanced by a history of a recent upper respiratory tract infection, a positive throat culture, or a positive antistreptococcal antibody test.
TABLE 35F.2 Revised Jones criteria for the diagnosis of acute rheumatic fever, 1992 | |||
|---|---|---|---|
|
Rheumatic carditis can involve the endocardium, myocardium, or pericardium. Most commonly, there is variable involvement of the cardiac valves, particularly the mitral valve and its subvalvular apparatus, including papillary muscles and their attached chordae tendineae. Severe clinical decompensation is the exception rather than the rule. The most feared consequence of this pathologic process is chronic scarring leading to mitral, and potentially aortic, valvular stenosis and/or regurgitation.
Treatment in mild cases is usually with high doses of aspirin (54




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree